Clinical Summary
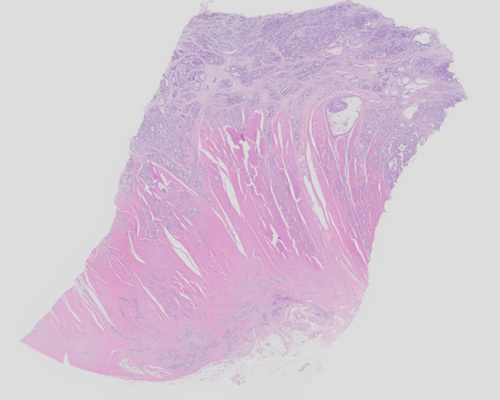
A 77-year-old woman presents with longstanding abdominal pain and distension. A computed tomography scan demonstrates a mass involving the stomach. The excision specimen shows a 7 cm elevated, centrally ulcerated tumor that invades the muscularis propria. The tumor is gray-white, homogenous, and infiltrative.
Master List of Diagnoses
- Gastric xanthoma
- Gastrointestinal stromal tumor, epithelioid type
- Malignant gastrointestinal neuroectodermal tumor
- Metastatic melanoma
- Primary gastric adenocarcinoma
Archive Case and Diagnosis
This case first appeared as Performance Improvement Program in Surgical Pathology (PIP) 2021, Case 01, and is primary gastric adenocarcinoma of the stomach. The information provided in this case was accurate and correct at the time of publication in 2021. Any changes in terminology since the time of publication may not be reflected in this case.
Criteria for Diagnosis and Comments
H&E-stained sections reveal an infiltrative tumor, composed of variably sized glandular structures infiltrating into the submucosa and muscularis propria. At higher magnification, the invasive glands show mitoses, moderate to marked pleomorphism, and granular chromatin with prominent nucleoli. Immunohistochemical stains of the tumor cells show positivity for CK7, CK20, and CDX2. The tumor cells are negative for GATA3, TTF1, and S100. Overall, these features are consistent with a primary adenocarcinoma of the stomach.
Although the incidence of gastric cancer is steadily declining, it remains the sixth-most commonly diagnosed cancer and the third-most common cause of cancer-related death worldwide. It is most prevalent in Eastern and Central Asia and Latin America. More prevalent in males, gastric cancer results from a combination of environmental factors and accumulation of specific genetic alterations. Studies have shown that the strongest risk factor for developing gastric cancer is infection by Helicobacter pylori. Other important risk factors include diet and smoking. The dietary links include high consumption of meat, smoked meats, and salt-preserved foods. The increased awareness of H. pylori, nutrition, and lifestyle choices has helped decrease the overall worldwide incidence of gastric cancer.
Gastric carcinoma can be categorized as early gastric cancers and advanced gastric cancers. Early gastric cancers are defined as an invasive adenocarcinoma limited to the mucosa or submucosa, independent of lymph node status. This implies not only the limited extent of the disease but also an early stage in development and a less-aggressive neoplasm. Early gastric carcinomas are typically located in the antrum, around the incisura, with predominance in the lesser curvature. Advanced gastric cancers are defined as invading into the muscularis propria, and thus a later stage in development and a more-aggressive neoplasm. Advanced gastric adenocarcinomas frequently involve the lesser curvature and can occur in the proximal stomach. The gross appearance and growth patterns can be varied. The incidence of nodal metastasis increases with depth of invasion. Gastric carcinomas can spread by peritoneal dissemination or direct extension.
Histologically, gastric carcinoma demonstrates marked heterogeneity at the architectural and cytologic level, often with mixed histologic elements. In the past, histologic classification was largely based on Laurén classification criteria, in which there were two major types, intestinal-type and diffuse-type adenocarcinoma, and an indeterminate/unclassified minor type. However, the 2019 World Health Organization (WHO) classification recognizes several morphological subtypes, including tubular, papillary, poorly cohesive, and mucinous variants. The intestinal type from the Laurén system encompasses both papillary and tubular types defined by the WHO, while the diffuse type from the Laurén system encompasses mucinous and poorly cohesive types defined by the WHO.
More than 90% of gastric carcinomas are sporadic; however, familial gastric cancers have been identified as well and are associated with specific gene mutations. There are many syndromes associated with increased risk, including Peutz-Jeghers syndrome, Lynch syndrome, juvenile polyposis, Li–Fraumeni syndrome, and most notably hereditary diffuse gastric cancer syndrome (HDGC). HDGC is an autosomal-dominant cancer susceptibility syndrome that is characterized by diffuse-type gastric cancer and invasive lobular breast cancer, resulting from a germline mutation of the CDH1 gene, which leads to a pure diffuse-type/poorly cohesive adenocarcinoma. Carcinomas from HDGC tend to arise in a younger patient population (often as young as teenagers) and carry a poor prognosis.
The College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology recommend that patients with advanced-stage gastric cancer undergo HER2 protein expression evaluation, as targeted therapy is available. ERBB2 is a proto-oncogene located on chromosome 17 that encodes the HER2 protein, and overexpression/amplification is present in 9% - 38% of patients with gastric cancer. HER2 positivity is associated with a poor prognosis, but many trials have shown prolonged survival in patients treated with anti-HER2 therapy.
HER2 analysis can be done on either a biopsy or resection specimen. In larger biopsies or resections, staining should be present in 10% of tumor cells. In smaller specimens, there should be a cluster of 5 or more positive neoplastic cells. Basolateral or lateral staining in a membranous pattern is interpreted as positive. Unlike breast cancers, incomplete staining is frequently observed. The scoring system ranges from 0 - 3+. A score of 0 or 1+ is deemed negative, 2+ is equivocal, and 3+ is positive. If the immunohistochemistry results are equivocal, then in situ hybridization (ISH) is done next to identify the presence or absence of gene amplification. To be considered positive, 20 cohesive tumor cells need to have an amplification ratio of 2.0 or higher. The current recommendation is treatment with trastuzumab for patients with positive immunohistochemistry (3+) or with equivocal immunohistochemistry and evidence of HER2 gene amplification by ISH.
Gastric xanthoma may be a consideration in certain cases, because the foamy cytoplasm of the histiocytes can appear histologically similar to the diffuse type of gastric carcinoma. However, there are stark differences that allow it to be easily ruled out. Gastric xanthomas do not contain atypical nuclei and mitoses, and they do not alter or destroy the mucosal architecture, which are features seen in gastric adenocarcinoma. Gastric adenocarcinoma tumor cells are cytokeratin positive and CD68 negative; the opposite pattern is seen in gastric xanthoma.
Gastrointestinal stromal tumors, epithelioid type have round cells with clear to eosinophilic cytoplasm arranged in sheets or nests, which can also appear histologically similar to gastric adenocarcinoma. These tumors are positive for DOG1, CD117, and CD34, which helps to differentiate them from gastric adenocarcinoma, which is negative for these markers. S100 would typically be negative in both tumors.
Malignant gastrointestinal neuroectodermal tumor, also known as clear cell sarcoma-like tumor of the gastrointestinal tract, is a rare mesenchymal neoplasm that frequently presents as sheets, nests, or papillary structures of oval/spindled mesenchymal cells with clear or eosinophilic cytoplasm, with visible nucleoli and often mitoses. This can mimic the morphology of a primary adenocarcinoma, but its positivity for S100, SOX10, and synaptophysin can help establish the diagnosis.
Metastatic melanoma has the ability to look morphologically similar to anything. It can be ruled out with immunohistochemistry, as it will be positive for S100, SOX10, and Melan-A, and negative for keratins.
Metastatic adenocarcinoma of the breast and the lung are both important differential diagnoses in this case. Both of these have similar architecture to gastric carcinoma. Morphology alone is insufficient in distinguishing these entities. Immunohistochemical stains can provide a definitive answer. TTF1 and Napsin A will generally be positive in adenocarcinoma of the lung, but negative in gastric carcinoma. For metastatic adenocarcinoma of the breast, CK7 can be positive in both entities, but strong and diffuse GATA3 and ER positivity would be suggestive of breast cancer.
Supplementary Questions
-
Hereditary diffuse gastric cancer syndrome is most commonly associated with a germline mutation in which of the following genes?
-
CDH1
-
EWSR1
-
MLH1
-
STK11
-
VHL
-
-
Which of the following immunohistochemical profiles is most characteristic of a gastrointestinal stromal tumor?
-
DOG1-, CD117-, S100+, CD34-
-
DOG1-, CD117+, S100+, CD34+
-
DOG1+, CD117-, S100-, CD34-
-
DOG1+, CD117+, S100-, CD34+
-
DOG1+, CD117+, S100+, CD34+
-
-
A 45-year-old man presents with advanced gastric adenocarcinoma, and HER2 testing is performed. Which of the following is true?
-
A HER2 gene amplification ratio of 1.5 is considered positive.
-
A HER2 immunohistochemistry score of 1+ is considered equivocal.
-
HER2 positivity makes the patient eligible for rituximab therapy.
-
HER2 testing is only indicated in breast cancer and not recommended in this setting.
-
In resection specimens, strong basolateral membranous reactivity (IHC) in 10% or more of tumor cells is considered positive.
-
References
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 2010;376(9742):687-697.
- College of American Pathologists. Template for reporting results of HER2 (ERBB2) biomarker testing of specimens from patients with adenocarcinoma of the stomach or gastroesophageal junction. V1.0.0.1 Published June 2017. Accessed February 19, 2020. https://documents.cap.org/protocols/cp-gastric-HER2biomarker17-1001.pdf
- Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251-261.
- Lokuhetty D, White V, Watanabe R, Cree I. Digestive system tumours, WHO Classification of Tumours, 5th ed. IARC press; 2019.
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26-38.
- Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: A practical approach. Mod Pathol. 2012;25(5):637-650.
- Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248.
- van der Post RS, Carneiro F. Emerging concepts in gastric neoplasia: heritable gastric cancers and polyposis disorders. Surg Pathol Clin. 2017;10(4):931-945.
- Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23(4):713-739.
Answer Key
- Hereditary diffuse gastric cancer syndrome is most commonly associated with a germline mutation in which of the following genes?
- A. CDH1
- B. EWSR1
- C. MLH1
- D. STK11
- E. VHL
- Which of the following immunohistochemical profiles is most characteristic of a gastrointestinal stromal tumor?
- A. DOG1-, CD117-, S100+, CD34-
- B. DOG1-, CD117+, S100+, CD34+
- C. DOG1+, CD117-, S100-, CD34-
- D. DOG1+, CD117+, S100-, CD34+
- E. DOG1+, CD117+, S100+, CD34+
- A 45-year-old man presents with advanced gastric adenocarcinoma, and HER2 testing is performed. Which of the following is true?
- A. A HER2 gene amplification ratio of 1.5 is considered positive.
- B. A HER2 immunohistochemistry score of 1+ is considered equivocal.
- C. HER2 positivity makes the patient eligible for rituximab therapy.
- D. HER2 testing is only indicated in breast cancer and not recommended in this setting.
- E. In resection specimens, strong basolateral membranous reactivity (IHC) in 10% or more of tumor cells is considered positive.