- Home
- Member Resources
- Councils and Committees
- Point of Care Testing Topic Center
Promoting the accuracy, precision, and quality of point of care testing (POCT) results.
Frequently Asked Questions Regarding Activated Clotted Time Cartridge Shortages
The Point of Care Testing and the Hemostasis and Thrombosis Committees discussed the testing alternatives and mitigation strategies in the event of reagent shortages for laboratories and point of care testing (POCT) sites who offer Activated Clotting Time (ACT) testing.
ACT is often needed to support surgeries/procedures where significant and rapid anticoagulation is required, such as coronary artery bypass graft (CABG), coronary stent placements, vascular surgery, extra corporeal membrane oxygenation (ECMO), etc. These procedures often require rapid turnaround time (TAT) and testing options that have wide analytic ranges to support the high levels of anticoagulation.
Frequently Asked Questions
- Open all
- Close all
Central laboratories may offer anti-Xa testing for monitoring unfractionated heparin therapy (UFH) in hospitalized patients. However, due to requirements for specimen transport, preparation (centrifugation of whole blood to obtain plasma), and testing time, central laboratories may not be able to meet the rapid turnaround requirements of cardiac and vascular procedures. In addition, the analytical measurement range of anti-Xa tests is often insufficient to measure the high heparin concentrations used in these procedures, which are typically much higher than those used for the treatment of venous thromboembolism (VTE). Central lab or point-of-care (POC) activated partial thromboplastin time (aPTT) measurements are typically not appropriate for anticoagulation monitoring during these procedures due to high sensitivity to the drugs and concentrations being used, non-specificity for drug effect, and lack of established target ranges, in addition to TAT concerns.
The anti-Xa assay is used to monitor therapeutic unfractionated heparin (UFH) with a range of 0.3 – 0.7 IU/mL, used for treatment of venous thromboembolism (VTE). The level of heparin anticoagulation used in many cardiac and vascular procedures (therapeutic heparin ranges of 1 IU/mL and much greater) will exceed the analytic measurement range of the anti-Xa assay. Extending the range via dilutions would not only require validation and categorization as an FDA modified test, but it is not recommended by many vendors of the anti-Xa assays due to non-linearity of diluted specimens. Thus, validating dilutions to extend the clinical reportable range may not be possible for many anti-Xa assays on the market. If the laboratory anti-Xa assay has a reportable range appropriate for the procedure in question and the laboratory is able to meet expected turnaround times, anti-Xa monitoring may be an option.
Additionally, variable correlations between ACT instruments and anti-Xa assay measurements must be considered, particularly when developing local target values Temporarily changing monitoring methods and target values may create significant patient care issues for the clinical services and should be considered with caution.
Several vendors do offer point of care ACT assays. Implementing new ACT assays at the point of care can be a complex and time-consuming process, not only does it require validation but since ACT values/targets differ by vendor and cartridge type, and alternate vendors have various cartridges options for low and high heparin ranges, there can be a significant educational hurdle and risk of confusion for clinical services. Literature reports only moderate correlation between POCT ACT instruments along with different sensitivities and specificities comparted to the anit-Xa assay methodology (1). Even different cartridges from the same vendor may recover different ACT results from the same heparinized specimen and must be carefully validated.
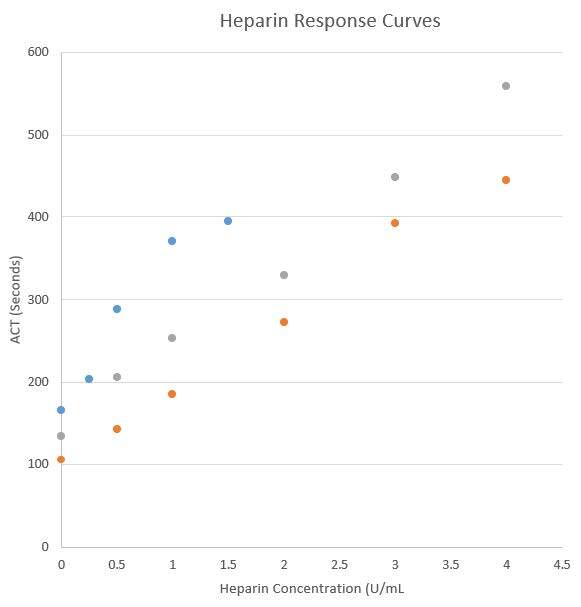
Below is an example from a single laboratory’s experience with 2 different ACT POCT cartridges from a single vendor (data not published).
Various mitigation strategies may be considered and include:
- Close communication with the vendor to get reliable estimates of reagent availability and timing.
- Management of cartridge usage and testing strategies to eliminate unnecessary testing.
- Determine what testing (for example anti-Xa or aPTT ) if any can be performed in the main laboratory with accelerated TAT and understand the ramifications for the clinical services of this change in testing and target values.
- Consider validation of a different vendor’s ACT instrument or a different cartridge from the same vendor if resources and time allow.
- As a very last resort, consider reducing elective cardiac and vascular cases or using alternative anticoagulation that does not require monitoring.
Reference
Falter F, MacDonald S, Matthews C, Kemna E, Cañameres J, Besser M. Evaluation of Point-of-Care ACT Coagulometers and Anti-Xa Activity During Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2020;34(11):2921-2927. doi:10.1053/j.jvca.2020.06.027
Point of Care Testing Toolkit
This toolkit for laboratory directors of POCT testing has been developed by the College of American Pathology (CAP) POCT Resource Committee as a service to CAP members and the laboratory community. It is intended to be a resource for any pathologist who wants to learn about POCT or who has responsibility to guide or direct POCT.
The POCT Toolkit is available digitally at ebooks.cap.org.
The toolkit contains the following chapters:
- Introduction & Definitions
- Advantages & Disadvantages
- History
- Current & Projected Technology
- References Tools
- Toolkit Authors
- Pathologist Roles
- Pathologist as Laboratory Director
- Pathologist as Clinical Consultant
- Pathologist’s Regulatory Role
- Pathologist as Technical Consultant
Articles on POCT
Visit CAP TODAY to access the following articles:
- An Uneasy Dance With POC Glucose in the ICU (October 2013)
Join the Point of Care Testing Committee
Learn more about our committee activities and how to join the Point of Care Testing Committee.