- Home
- Member Resources
- Councils and Committees
- Immunohistochemistry Topic Center
- Frequently Asked Questions from Immunohistochemistry Laboratories
Maintaining and expanding a test menu in immunohistochemistry can be complex. The following FAQs will address issues related to validation of tests that are new to a laboratory, as well as potential responses to test performance monitoring, especially in the setting of proficiency testing and/or other external quality assessment. Hypothetical examples of process improvement assessments are described, which are based on CAP proficiency testing participant queries and the experiences of CAP Immunohistochemistry Committee members.
- Open all
- Close all
The general concept of “validation (lower case v)” refers to measures taken by a laboratory to ensure that an assay performs as expected before putting it into clinical use. In the context of immunohistochemistry, the semantic distinction between Validation (capital V) and Verification has to do with whether the new stain is FDA approved/cleared for in-vitro diagnostics or not. Laboratory developed tests (non-FDA approved) require Validation and FDA approved tests require Verification. Pragmatically, the difference is really in the stringency of the evidence needed to support the use of a new test in a laboratory.
Validation refers to the process by which the entire assay is executed and confirmed to perform acceptably compared to a pre-determined gold-standard result. Stated another way, validation is “confirmation, through the provision of objective evidence, that requirements for a specific intended use or application have been fulfilled (ISO 9000).” A laboratory must validate laboratory-developed immunohistochemical tests (LDTs) before placing them into use. A laboratory must re-validate tests if assay conditions change. Subsequent FAQ’s will address the nature and extent of the validation cohorts.
Verification refers to the process of ensuring the performance of FDA-approved or cleared assays. Because significant work has already been done by the manufacturer to gain regulatory approval, in theory, verification could be less extensive. If the vendor provides specific instructions on assay verification in the kit instructions, following those would suffice. Many kit instructions only refer to the CAP Center Guideline on Analytic Validation of Immunohistochemical Assays, which does not specify a less stringent process for verification.1 However, in the absence of specific guidance, verification could take the form of validation.
For HER2 immunohistochemistry, however, the ASCO/CAP guidelines do make specific recommendations for Validation and Verification - Verification should include 20 known positive and 20 known negative cases and Validation should include 40 known positive and 40 known negative cases.2 Specific guidelines such as these supersede even the CAP Center Guideline on Analytic Validation of Immunohistochemical Assays.
References
- Fitzgibbons PL, Bradley LA, Fatheree LA, et al. College of American Pathologists Pathology and Laboratory Quality Center. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014 Nov;138(11):1432-43.
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018 Nov;142(11):1364-1382
The IHC lab director has determined that testing volumes support the financial/clinical viability of new stain and confirmed the resources needed to supply materials for validation and the ongoing need for control tissues are available, the steps for validation and maintenance are:
- Optimization:
- Select from commercially available clones (clone specific data from NordiQC assessments and CAP surveys can be a valuable tool in this task)
- Identify an appropriate tissue for optimization (tissue with known expression of target antigen and a negative control)
- Follow manufacturer recommended protocol and review stain results
- If needed, adjust conditions of the reaction (dilution, incubation, pretreatment parameters, etc.) and review stain results iteratively until an optimal staining pattern is achieved
- Note, optimization is not necessary for FDA approved/cleared reagents. They must be used according to the manufacturer’s directions.
- Validation/Verification:
- Determine the appropriate number of known positive and negative cases for the validation plan
- Predictive markers require more cases to be tested - 20 positive and 20 negative per the CAP IHC Validation Guideline compared to 10 positive and 10 negative for non-predictive markers1
- Rare analytes or low frequency antigens may prove a challenge and require collaboration with other labs, use of cell lines, or other creative strategies
- The medical director ultimately determines how many cases to include, but if the number is less than what is recommended above it should be documented with a scientifically supported justification for this
- Identify known positive and negative cases
- Including a range of expression levels is ideal
- Gold standards for positive and negative vary, but include
- Prior stain result using a validated method
- A validated non-IHC method (eg. ISH or molecular)
- A tissue or tumor type defined by specific protein expression
- Stain and interpret the validation plan cases
- Using tissue arrays, multi-tissue blocks or multiple sections per slide can reduce reagent use and cost
- Analyze results:
- Typically an overall concordance threshold of 90% is used, but any discordant results should be scrutinized
- Concordance among the positive and negative cohorts alone should be reviewed
- ‘Missing’ positive cases but not negative cases suggests the sensitivity may be too low and ”missing” negative cases but not positive cases suggests a specificity problem
- ‘Missing’ both negative and positive cases suggests a general problem with the assay or validation case selection
- Determine the appropriate number of known positive and negative cases for the validation plan
- Clinical ‘go-live’ for the new stain
- Communicate the new availability and describe the utility of the stain to help guide appropriate ordering
- Assay Maintenance
- Carry out ongoing monitoring:
- Encourage feedback from colleagues, sharing cases where results differ from the expected
- Ideally track positive and negative rates for predictive markers to compare with benchmarks
- Continue lot-to-lot stain comparisons
- Ensure appropriate control block supply
- Enroll in Proficiency Testing or Alternative Assessments with another lab
- Carefully review results for trends suggesting the assay may be ‘drifting’ or lacking appropriate sensitivity or specificity
- Carry out ongoing monitoring:
References
- Fitzgibbons PL, Bradley LA, Fatheree LA, et al. College of American Pathologists Pathology and Laboratory Quality Center. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014 Nov;138(11):1432-43.
Adding to a laboratory’s test menu is a multifaceted decision, which must consider clinical utility and demand (both significance of the result and required timeliness), cost, and resources required to onboard and perform the test. Pathologists may request that a laboratory validate a recently published diagnostic marker or clinicians may request that a laboratory validate a predictive marker that would yield important information about the benefit of a potential therapeutic.1
When a laboratory medical director receives a request, a thorough investigation is needed before a laboratory commits to assay validation. This investigation is often the most time intensive piece to bringing up a new IHC assay. However, a thorough investigation is well worth the time investment as it can provide valuable information and potentially cost and time savings.
It may be helpful to have a standard document in the laboratory to guide the pre-validation investigation (download a sample pre-validation template). This document or worksheet should be modified as appropriate for the laboratory and might include prompts to consider:
- In-depth review of primary literature and other resources, including NordiQC (www.nordiqc.org), which publishes detailed, clone-specific external quality assessment data and makes autostainer platform-specific protocol recommendations; www.ihcfaq.com, a free online complement to the textbook Handbook of Practical Immunohistochemistry (Springer 2015); www.pathologyoutlines.com, which curates content on diagnostic applications of immunohistochemical markers; IHC data sheets (aka “product inserts”), which are available, typically in PDF form, for each vendors formulation (monoclonal or polyclonal) for a given marker. The goal of this review is to identify or confirm, as needed:
- Antibody vendor
- Antibody clonality (monoclonal or polyclonal; specific clone name and host [typically mouse or rabbit], if monoclonal)
- Potential assay parameter considerations such as antibody dilution, ready-to-use options, and additional protocol considerations
- Possible additional assay diagnostic applications (i.e., “purposes”) beyond the initial request
- Performance of the chosen clone in normal and diseased tissues with an emphasis on differential diagnostic considerations (i.e., theoretical diagnostic sensitivity and specificity)
- Known performance issues associated with specific clones
- Appropriate gold-standard comparison method(s) by which assay concordance will be determined (e.g., diagnostic accuracy based on the published literature; results of orthogonal testing including molecular testing or FISH; parallel testing in a laboratory having already validated a given marker)
- Discussion with pathologists and/or clinicians involved in ordering the IHC assay to confirm, as needed:
- Clone preference
- Anticipated applications (diagnostic, prognostic, and/or predictive) as different purposes may impact the nature and extent of the validation
- Projected volumes
- How test results will be reported
- Discussion with laboratory staff regarding:
- Confirmation that sufficient material is available for validation, including material from cases that will be in the diagnostic differential (i.e., “expected negative” cases)
- If sufficient material is unavailable in house, how will it be obtained?
- Sufficient source of anticipated control tissue
- Are there laboratory policies (restriction against use of research use only [RUO] antibodies), purchasing agreements, or preferred vendor relationships that constrain antibody clone choice?
- How will anticipated test volumes and test protocol impact current work flow?
- Which testing platform (i.e., autostainer) will be used, if multiple are available
- Are there IT issues related to result reporting and billing (e.g., disclaimer modification required?)
Depending on the organization and preference of the laboratory, it is recommended that the above be reviewed and discussed in meeting(s) with all relevant stakeholders present, including, as applicable for the laboratory, laboratory medical director, IHC technical specialist or laboratory subsection director, and/or lead immunohistochemistry laboratory staff. Any stakeholder may express concerns to committing to the validation. If these cannot be resolved, continuing with a validation may not make sense for the laboratory.
Reference
- Torlakovic EE, Cheung CC, D'Arrigo C, Dietel M, et al. From the International Society for Immunohistochemistry and Molecular Morphology (ISIMM) and International Quality Network for Pathology (IQN Path). Evolution of Quality Assurance for Clinical Immunohistochemistry in the Era of Precision Medicine. Part 3: Technical Validation of Immunohistochemistry (IHC) Assays in Clinical IHC Laboratories. Appl Immunohistochem Mol Morphol. 2017 Mar;25(3):151-159.
After pre-validation investigation is complete, the chosen antibody must be obtained by the laboratory and a validation cohort of paraffin blocks from relevant tissues must be identified and assembled (see question 6 for more details on validation cohorts).1 A subset of cases from the validation cohort is used for assay optimization. At least one block should be used, but it is ideal to use more than one to reflect the potential range of expression of the analyte and to mitigate against the impact of pre-analytic variables that might affect staining in any one tissue block. Expected positive and negative tissue types should be included - testing more than a few blocks is unnecessary in this step. Multi-tissue blocks or tissue microarrays are useful tools for optimization, if available.
The goal of assay optimization is to establish assay working conditions for the subsequent validation. Tissues are examined at different assay conditions to confirm reliable, appropriately localized, positive staining at an acceptable intensity while minimizing non-specific background staining (optimum “signal-to-noise” ratio).
Parameters to consider in the optimization include antigen retrieval type and duration, primary antibody dilution, primary antibody incubation duration, and detection incubation duration.2 Occasionally, if available, various staining platforms might be tried. Many antibodies come as ready-to-use (i.e., prediluted) formulations. While these are certainly easier reagents to use, these preparations are not always optimal in the laboratory due to available staining platforms, potential fixation concerns and/or other conditions. For those received as concentrates, the primary antibody dilution may be selected based on published literature, reputable IHC websites (e.g., NordiQC), manufacturer’s recommendations, or personal communications with labs that already successfully use the antibody. In the absence of such guidance, a starting point of 1 μg/mL is reasonable (e.g., 1:100 if the antibody stock solution is at a concentration of 100 μg/mL).
For the initial optimization, if using a concentrate, it is useful to examine the antibody at a given dilution at two or more antigen retrieval conditions (typically at low and high pH heat-induced epitope retrieval). It is also useful to examine two-fold serial dilutions above and below your selected starting point with at least one antigen retrieval condition (download a sample Antibody Optimization template).
With this initial experiment, one set of assay parameters is often associated with a better signal-to-noise ratio (e.g., stronger signal at a given dilution; no/less background staining at a given dilution—ideally both but sometimes one at odds with the other). Additional refining conditions can be assessed based on these initial results.
Laboratories typically have a preferred or most commonly employed (baseline) antigen retrieval type and duration, primary antibody incubation conditions, and detection chemistry incubation durations. Some of these baseline conditions may be based on optimizing use of instrument capacity or other whole-lab workflow processes. However, these can and if necessary should be further optimized as needed. Durations can be lengthened to enhance signal at a given dilution (though possibly at the expense of background staining) or shortened to mitigate background (though possibly at the expense of signal). Occasionally, a background-reducing blocking step is needed to deal with non-specific staining, though most issues with background can be handled by optimizing retrieval, primary antibody dilution, and timing. Sometimes signal amplification is necessary, however, that is often at the cost of occasional granular non-specific staining.
The optimized protocol is subsequently applied to the validation cohort. Slides are reviewed by the medical director (or another pathologist, i.e. the proponent of the stain) and concordance with the pre-determined gold standard result (e.g., morphologic diagnosis, results of panel of established IHC markers supporting the diagnosis, available molecular studies that are consistent with the diagnosis) is determined. If acceptable concordance is not achieved, discrepancies should be investigated (e.g., clerical error or the possibility that a “positive” case was misclassified as a “negative” case or vice versa due to prior diagnostic error, use of orthogonal methodologies with differing sensitivities, etc). Sometimes, discrepancies can be resolved by sending unstained slides from discrepant cases to an independent laboratory that has already validated the test to produce a tie-breaker result. Sometimes assay conditions need to be re-optimized and staining of the validation cohort has to be repeated with the re-optimized conditions.
When acceptable concordance has been achieved in a sufficient number of cases based on the intended use of the marker, the medical director has completed necessary documentation, and other necessary processes are implemented in the laboratory (e.g., identification of control material, creation of an electronic order for the stain, report disclaimers modified if needed), the assay may be performed, interpreted, and resulted on patient samples (download a sample Antibody Validation Checklist template).
References
- Hsi ED. A practical approach for evaluating new antibodies in the clinical immunohistochemistry laboratory. Arch Pathol Lab Med. 2001 Feb;125(2):289-94.
- https://www.labome.com/method/Antibody-Dilution-and-Antibody-Titer.html
Diagnostic markers are performed to support or refute a morphologic impression. Because only rare IHC markers are specific for a certain disease, IHC stains are often performed as part of a panel. The diagnostic pathologist incorporates these results into the histomorphologic diagnosis (i.e., while they are often essential, they are ancillary to the diagnosis). Examples include keratin, S100, SOX10, desmin, etc.1
For a given tumor type, prognostic markers provide probabilistic information on patient outcome (e.g., likelihood of tumor recurrence, likelihood of survival at a specified follow up interval). Examples include Ki-67 in some conditions.
Predictive markers provide information on the likelihood of a patient responding to a specific therapy. This information is independent of morphology. Predictive markers can be performed concurrent to the diagnosis, but they may also be ordered after the fact—even years later in the setting of tumor recurrence/metastasis or failure of other treatment lines. Predictive markers are performed as reflex testing at some institutions for certain diseases/stage of disease. While at other institutions, predictive markers are chosen and requested by clinicians, in particular oncologists, who make therapy decisions based on the results.
The results of predictive marker IHC testing may drive clinical decision making independent of other factors, especially including tumor morphology in some situations. In addition, because any administered therapeutic is associated with risk of side effect, some of which could be life-threatening, the performance and resulting of predictive marker testing receive greater scrutiny in terms of validation and external quality assessment than diagnostic markers. Examples include PD-L1, estrogen receptor (which also can be a diagnostic marker), and HER2.
Some markers fulfill more than one of these roles, sometimes even in the context of a single case. In some instances, a marker that is merely diagnostic in one setting is predictive in another. Knowing which of these purposes a given marker will be used for is important as it dictates the extent of validation and the necessity of external quality assessment.
Reference
- Cheung C. D’Arrigo C. Dietel M. et al. Evolution of Quality Assurance for Clinical Immunohistochemistry in the Era of Precision Medicine: Part 1: Fit-for-Purpose Approach to Classification of Clinical Immunohistochemistry Biomarkers. Appl Immunohistochem Mol Morphol. 2017. Dec;25(1)4-11.
- Diagnostic and prognostic markers: minimum of 10 expected positive and 10 expected negative cases; concordance target ≥90%
- Predictive markers (other than ER/HER2): minimum of 20 expected positive and 20 expected negative cases; concordance target ≥90%
At present, there are no published guidelines as to whether predictive markers should be validated by marker or by tumor type on which predictive testing is being performed or by scoring system used to determine a result that is predictive of response to therapy. It is a growing opinion that full validations of predictive markers by tumor type is overly burdensome. However, while it is generally agreed that the IHC antibody clones are tumor agnostic, lack of pathologist reproducibility in read-out and interpretation combined with existence of multiple scoring criteria for certain predictive markers (PD-L1) require careful consideration when it comes to generating published guidelines.
- ER/HER2 laboratory developed tests: minimum of 40 expected positive and 40 expected negative cases; concordance target for ER ≥95% for expected negative and ≥90% for expected positive cases; concordance target for HER2 ≥95%
- ER/HER2 FDA-approved assays (verification): 20 expected positive and 20 expected negative cases with same concordance targets as for laboratory developed tests.
The parameters for the diagnostic and non-breast predictive markers are according to the CAP Center Guideline “Principles of Analytic Validation of Immunohistochemical Assays.”1 The parameters for HER2 were initially established in the 2007 and 2014 ASCO/CAP HER2 Testing Guidelines, which were affirmed in 2018.2 The parameters for ER were initially established by the 2010 ER/PR Testing Guideline, which referred to a white paper from Fitzgibbons et al;3 these were affirmed in 2020.4 The extent of validation of a prognostic marker is not explicitly stated in the CAP Center Guideline, though it should be at least as rigorous as for a diagnostic marker.
At times, more than the minimum number of cases may be needed or desired to achieve the required concordance rate and/or to assure that the assay performs acceptably. The expected positives in the validation cohort should encompass all the intended purposes of the assay and, if possible, should include cases demonstrating a range of antigen expression (i.e., they should not only include strong positives). The expected negatives should include cases that are differential diagnostic considerations for the cases in the expected positive cohort, to ensure the assay achieves sufficient diagnostic sensitivity and specificity.
If an assay is intended for diagnostic and predictive purposes, the extent of the validation should be at least as extensive as for a predictive marker.5
In addition to the above, it is desirable to run the assay in multiple normal tissues (a multi-tissue block or TMA is a useful tool for this purpose) to look for aberrant staining, which might not be reported in the literature (this is referred to as extended analytical specificity). For new IHC antibodies with limited literature, it is important to monitor the literature over time as reported test characteristics tend to change over time with the accrual of more data.
References
- Fitzgibbons PL, Bradley LA, Fatheree LA, et al. College of American Pathologists Pathology and Laboratory Quality Center. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014 Nov;138(11):1432-43.
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018 Nov;142(11):1364-1382
- Fitzgibbons PL, Murphy DA, Hammond ME, Allred DC, Valenstein PN. Recommendations for validating estrogen and progesterone receptor immunohistochemistry assays. Arch Pathol Lab Med. 2010 Jun;134(6):930-5.
- Allison KH, Hammond MEH, Dowsett M. et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med. 2020 May;144(5):545-563
- Cheung CC, D'Arrigo C, Dietel M, et al. From the International Society for Immunohistochemistry and Molecular Morphology (ISIMM) and International Quality Network for Pathology (IQN Path). Evolution of Quality Assurance for Clinical Immunohistochemistry in the Era of Precision Medicine: Part 4: Tissue Tools for Quality Assurance in Immunohistochemistry. Appl Immunohistochem Mol Morphol. 2017 Apr;25(4):227-230.
There are several options to consider in selecting a gold standard comparator method for an immunohistochemical validation. Practically and economically speaking, if the antibody to be validated is currently being performed as a send-out IHC test at another laboratory, it is usually most straightforward to use the results of prior send-out tests as the comparator method. Demonstrating concordance with positivity rates reported in the medical literature is also acceptable.
For certain tests, comparison with a molecular gold standard may be an option. For example, validation of point mutation specific antibody clones (e.g., IDH1 R132H or BRAF V600E) may be done compared to results of a molecular assay confirming the point mutation. However, it is necessary to understand the output of the molecular assay in advance – using a BRAF V600 molecular assay to define “positive” cases may result in inclusion of cases in which the substituted amino acid is not glutamic acid (non-E) but instead a different amino acid resulting in conformational changes that are unrecognized by the BRAF V600E clone. This could result in an insufficient level of concordance to complete validation.
Publications describing methods of antibody validation have additionally advocated for use of one of three options: 1) determining concordance with an alternative antibody that binds to a non-overlapping epitope of the antigen, 2) use of orthogonal methods, and 3) use of a genetic method of validation.1-3 The third option includes the potential to validate against cell lines with established quantitative levels of protein expression. Although not presently widely available, cell line material with known expression levels are likely to become an important part of antibody validation in the future.
References
- Fitzgibbons PL, Bradley LA, Fatheree LA, et al. College of American Pathologists Pathology and Laboratory Quality Center. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014 Nov;138(11):1432-43.
- MacNeil T, Vathiotis IA, Martinez-Morilla S, et al. Antibody validation for protein expression on tissue slides: a protocol for immunohistochemistry. BioTechniques. 2020 Jun;69.
- Uhlen M, Bandrowski A, Carr S, et al. A proposal for validation of antibodies. Nature Methods. 2016 Oct;13(10):823-827.
As part of a laboratory’s validation process, careful consideration must be given to the feasibility of procuring not only enough positive and negative cases for the validation plan, but to also ensure a continuous supply of control tissue to maintain the assay. This is obviously a challenge for rare antigens, and there is not an easy answer for most labs.
Potential strategies for identifying a supply of these resources include obtaining tissue from other institutions or potentially outside vendors, identifying in-house cases shown to be positive using another validated methodology (FISH, molecular, etc.), or using engineered cell lines that were fixed in formalin after culturing for an appropriate period of time.
When partnering with other institutions it is important to understand how their pre-analytic process compares to your own for consistency.
If an initial effort to find enough cases to complete a planned validation falls short, a laboratory could start with a certain number of cases and add to the number in the course of a parallel testing strategy. Slides could be referred to another reference lab for a time and as positive results return those cases could be added to the validation set.
After validation, the importance of external quality assessment for rare antigens cannot be overstated. This is particularly true for predictive markers with low prevalence. Subscribing to the appropriate CAP proficiency test or other alternative assessment program develops trust in the assay performance. The CAP IHC committee prioritizes developing and expanding these types of survey and proficiency testing products, anticipating the critical need for these in the IHC lab community.
Immunocytochemical assays are tests performed using cytologic preparations as substrate (versus traditional formalin fixed paraffin embedded histologic sections). Cytologic preparations come in many forms, including formalin fixed paraffin embedded cell blocks, alcohol fixed paraffin embedded cells blocks, paraffin embedded cell block material fixed sequentially in alcohol and formalin, unstained liquid based preparations (alcohol fixatives), and air-dried cytologic smear specimens.
Rigorous recommendations for validation of immunocytochemical assays are currently lacking. It is generally considered best practice to include cytologic preparations in new antibody validations, if it is anticipated that the stain will be ordered on cytology specimens; however a specific number requirement for a validation is not specified at this time.
There are several factors to consider when thinking about control tissues, including tissue type(s), range(s) of antigen expression in tissue components, and availability/scarcity of blocks and/or tissue at your disposal.1-3
The question of whether to perform individual on-slide versus batch controls is also relevant to this discussion. A positive control must be utilized for each antibody performed. A routine negative control for each antibody is not required since polymer-based detection kits have obviated the reagent need for endogenous biotin checking and most patient samples include intrinsic negative control cells. A single control slide can be run for a batch of tests using the same antibody (“batch control”) or a control tissue section can be put onto each individual patient slide (“on-slide control”). On-slide controls are preferred whenever possible but are not required. On-slide controls should be clearly demarcated on the slide to prevent misinterpretation.
For some markers (e.g., ER), recommendations exist regarding types of control tissue to be used. The most recent ASCO/CAP ER/PR guidelines recommend a 4 tissue multi-tissue control block including: an ER strong positive tumor, an ER negative tumor, normal breast, and tonsil (as a reproducible ER low positive tissue type).3 For most remaining markers, specific tissue guidelines do not exist. In these situations, it is ideal to use reliably positive control tissue that can be easily obtained from discarded tissues or obtained commercially in an economical way. It is further preferred to use tissue types expressing normal constitutive or even low levels of antigen (low positive cases) so assay sensitivity can be confirmed with each run and false negative results avoided.
In addition to external control tissues, it is also recommended that pathologists routinely and systematically examine any available internal control cell types. Examination of the internal control in cases of ER immunohistochemistry is a required element in the CAP breast biomarker synoptic template, which aligns with recent ASCO/CAP guideline updates.3
References
- Fitzgibbons PL, Bradley LA, Fatheree LA, et al. College of American Pathologists Pathology and Laboratory Quality Center. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014 Nov;138(11):1432-43.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 5th edition. 2019. Elsevier. Chapter 1.
- Allison KH, Hammond MEH, Dowsett M. et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med. 2020 May;144(5):545-563
There are several reasons for ‘sunsetting’ an IHC assay. Most IHC assays slated for retirement have become diagnostically redundant, having been replaced by more sensitive and/or specific IHC markers or by alternative testing methods. When an assay no longer serves a useful clinical purpose and by remaining on the menu prevents a better stain from being used, it should be retired, even if resource costs are relatively low.
Infrequent ordering is another common reason for eliminating an IHC assay from the test menu. Given reagent costs incurred within the antibody expiration time window, it may be more cost effective to change an in-house test to a send-out test if it is rarely ordered.
Availability of antibody clones may change as manufacturers merge and fold over time. As mentioned before, it may prove impossible to maintain a supply of control tissues for some antibodies, necessitating removal from the test menu.
The CAP Accreditation Program has made changes to PT requirements for laboratories performing predictive marker testing using IHC for the 2023 program year.1 To align with the requirement changes we have made changes to the activity menu. Laboratories are required to review the new activities and make updates to their activity menu. The 2022 Checklist includes an update to COM.01520 - PT and Alternative Performance Assessment for IHC, ICC, and ISH Predictive Markers.
The updated IHC predictive marker PT requirements are as follows for breast ER, breast HER2, gastric HER2, and high sensitivity ALK:
- Laboratories performing both IHC staining and interpretation at the same laboratory must enroll in PT.
- Laboratories only performing predictive marker IHC staining (slides interpreted at a different laboratory) must perform alternative performance assessment.
- Laboratories only performing predictive marker IHC interpretations (slides are stained at a different laboratory) must perform alternative performance assessment
These PT requirement changes will reduce the burden of ordering the same PT for multiple laboratories in the same network/system and at the same time allow the CAP to monitor the accuracy of testing for additional predictive markers. Laboratories may continue to utilize PT to meet requirements for APA. Participation in CAP Immunohistochemistry Surveys satisfies this requirement. A list of currently available CAP IHC Surveys is available here.
For all other IHC predictive markers (MMR, PDL1, CD30, etc), semiannual alternative performance assessment is required, but CAP does not require proficiency testing for these analytes. Alternative performance assessment can be accomplished in many ways, and which option makes sense for a given laboratory is to be determined by the laboratory director.
Laboratories should review their ongoing quality plan annually to ensure they are enrolled in the appropriate Proficiency Testing Programs and as appropriate, are performing Alternative Performance Assessments.
References
- Newitt VN, et al. What's required in '23 for predictive markers? CAP TODAY. September 2022;36(9):27-28.
A written quality management plan is required of Anatomic Pathology (AP) laboratories by Laboratory Accreditation Programs. Although this requirement is for the entire AP laboratory, laboratory subsections stand to benefit from a similar, standardized approach focused on their subsection content.
Elements of a Quality Plan for the IHC laboratory could include a mission statement specific to the IHC lab that is in alignment with any mission statement of the laboratory and/or institution, identification of laboratory team members with definition of responsibilities, identification of IHC quality management committees with definition of responsibilities, identification of specific on-going QI projects and project team members, their responsibilities, and project timelines and/or a list of specific monitors including reporting schedule and reference benchmarks, assessment of risks, calendar of quality topics and/or monitored tests/projects that require regular, systematic data or literature review, data required for reporting to hospital/lab quality management committee, and incident reporting monitoring and results.1 An IHC specific process improvement assessment plan is an important component. Additional elements might include utilization data, results of pathologist read-out assessments (competency assessment), annual review of proficiency testing subscriptions, and future goals for the laboratory.
It is also helpful for the IHC laboratory to consider the IHC process comprehensively to identify pain points or points in the process where error is possible or likely or where inefficiency and waste can be minimized. This review can be started with a value stream map. When potential non-conforming events and the points in the process at which they might occur are identified, appropriate procedures can be put in place to prevent or detect the error quickly.
Ultimately, forming a quality plan for any part of the laboratory requires strong collaboration with quality assurance professionals and strong leadership from laboratory medical directors to make quality assurance a priority for the immunohistochemical laboratory.
References
- Zhai Q and Siegal GP, eds. Quality management in Anatomic Pathology Strategies for Assessment, Improvement, and Assurance. 2017 CAP Press. Northfield, IL.
Any concern about the performance of any assay in the laboratory should trigger at minimum an informal process improvement assessment. A single unacceptable response (one core on a 10 core TMA) due to a clerical error may not lead to significant change in the laboratory, but the cause of an unacceptable response must be determined, to the extent possible, and triaged appropriately by laboratory leadership. Conversely, investigation of a single unacceptable response could identify a situation requiring a complex improvement plan requiring assay re-validation. Therefore, review and assessment of all unacceptable responses, regardless of whether the laboratory achieves an overall acceptable score for the survey, is recommended.
An unsuccessful event indicates the laboratory did not achieve overall acceptable concordance with the intended responses (e.g., did not achieve a passing score). In this situation, a comprehensive process improvement assessment should be initiated with appropriate corrective action taken for each unacceptable result.
In case of an unsuccessful proficiency testing event, careful attention should also be given to the process improvement assessment step D3: interim containment action, depending on the assay in question. If the marker is a predictive marker, it may be appropriate to stop in-house testing until corrective action is implemented. The full process improvement assessment outline is described in the next question.
A process improvement assessment (PIA) is a standard, systematic approach used to identify pain points and sources of error, waste, or inefficiency in a process. Many methodologies derived from manufacturing-based industry exist to guide process improvement assessment and planning. Inasmuch as laboratory processes are similarly attempting to generate a product with minimal error and waste, these methodologies can be directly applied to IHC laboratory processes or applied with minor modifications. An intuitive process improvement method developed by Ford Motor Company in the 1980s is the Eight Disciplines problem solving method (8Ds).1 This process includes principles found in other methodologies, most notably Lean/Six Sigma.2-7
The 8D method uses the following steps:
- D0: Prepare and plan for the 8D process
- D1: Form a team
- D2: Describe the problem
- D3: Interim containment action (immediate steps to protect patients, if necessary)
- D4: Root cause analysis
- D5: Permanent corrective action (long term plan, corrective and preventive action plan [CAPA])
- D6: Implement and validate the permanent corrective action
- D7: Prevent recurrence
- D8: Closure and team celebration
Process improvement and planning tools exist to help complete steps of the 8D method:
D0: Prepare and plan for the 8D process
At the beginning of a process improvement assessment initial information is collected to estimate the required personnel and time for the project. It may be helpful to use problem assessment templates such as Fishbone or Pareto Diagrams to map the issues and questions to consider for problems identified by proficiency testing results or control failures.
At this step, and in step D3, careful consideration must be given to minimizing effect on patients due to the possible source(s) of error. If it initially appears that the problematic output from the lab could have significant negative effect on patients, then a decision about an interim containment plan (step D3) must be done in a timely manner and the project timeline must reflect this urgency.
In some laboratories, the need for an interim containment plan and timeline for implementation of corrective action may be guided by risk ranking and risk prioritization plans that use established patient safety harm categories and safety assessment code matrices.
Various project management tools can be used to organize and visually represent phases of the project. A Gantt chart (www.gantt.com) is essentially a horizontal bar chart that can be used to visually represent phases of a project and tasks scheduled over time. Gantt charts can be created using a template within Microsoft Excel (https://templates.office.com/en-us/simple-gantt-chart-tm16400962).
D1: Form a team
In this step, a multidisciplinary team representing relevant stakeholders is assembled. Responsibilities need to be clearly assigned – a team leader and, for major process improvements, a senior “champion” should be identified. The latter is someone with sufficient organizational clout to minimize obstacles that may come up in the course of process improvement. For a faulty breast predictive marker for example, the team could include breast pathologists or pathology chief, IHC medical director, breast surgeons and/or oncologists, and laboratory staff.
Tools are available to help identify process parts, stakeholders, and assign responsibilities such as a SIPOC diagram or responsibility assignment matrix https://www.projectsmart.co.uk/how-to-do-raci-charting-and-analysis.php
D2: Describe the problem
The problem definition may appear straightforward; however, it is generally advisable to approach problem definition in an open-minded manner driven by genuine curiosity. A common tool used for problem definition is called “5 Whys” – where one repeatedly self-questions explanations. For example, if the issue at hand is that the laboratory did not achieve acceptable concordance with the intended responses for a proficiency test:
Ask why #1:
Because, compared to the intended responses, we resulted in 3 of 10 cores on the TMA a response of negative when the intended response was positive.
Ask why #2:
Because we did not see staining in these cores.
Ask why #3:
Because it’s possible our assay is insufficiently sensitive to detect the protein of interest in these 3 cores.
Ask why #4:
Because our assay parameters do not align with those reported by the majority of participating laboratories using similar platforms.
Ask why #5:
No reason – we didn’t realize that our assay parameters were different than those used in other laboratories using similar platforms, and we failed to consider the parameters most commonly used in other laboratories using similar platforms when we performed the initial antibody validation. In this example, a problem definition may be: Our IHC assay appears insufficiently sensitive to detect low positive results, potentially due to suboptimal assay parameters.
Other tools used in this step may include simple flowcharts, Fishbone diagrams, Is/Is not comparison, or affinity diagrams.
D3: Describe the problem
Based on the problem definition, if necessary, a temporary, interim containment action should be verified and implemented. An interim containment plan is intended to be a preliminary stopgap and is often replaced by the permanent corrective action (step D5). In immunohistochemistry, the easiest interim containment plan is to stop performing the assay in-house and send-out material to a reference laboratory.
D4: Root cause analysis (RCA)
The root cause analysis (RCA) will take different forms with different tools applied, depending on the problem definition. The goal of a RCA is to determine the primary source of the error and the escape point, or the first point at which the error might have been detected but was not.
Tools such as failure mode and effect analysis (FMEA), fault tree analysis, or possibly a value stream map can be applied. Some tools from step D2 are also helpful in a RCA (5 Whys, Fishbone diagram). In the process of performing a RCA, additional potential sources of error may be identified – using the assay with insufficient sensitivity example, while considering all testing phases, it may surface that it was also the case that the particular TMA slide was not handled in the recommended manner prior to testing (possible global decrement in antigenicity) or that the pathologist read-out was near a subjective, difficult to reproduce threshold or that there was a simple clerical error and that the read-out pathologist selected the wrong bubble responses (negative, <1% when intended to select positive 1-10%). If other sources are identified, the problem definition and possible solutions can be further expanded.
It remains advisable to approach the RCA with an open-minded, genuine curiosity. While pursuing the root cause and escape point, it is imperative that team members and team leaders cultivate a non-pejorative, transparent team culture.
D5: Permanent corrective action (PCA)
The permanent corrective action is directed against the root cause and removes or alters the conditions that were responsible for the problem. Prior to selecting the permanent corrective action (PCA), acceptable performance criteria must be established, including any mandatory performance criteria, and effectiveness of the PCA must be demonstrated.
When there is a choice in regards to the PCA, the team leader must make efforts to make a balanced choice, and consider favoring choices that attend to the escape point as well, so that if error reoccurs, it will be captured at the escape point and effect on patients may be minimized. (The escape point for predictive markers is likely to be correlation with morphology (such as in breast), results of peer review or adjudication procedures, or quarterly quality monitoring reports.) Tools exist to assess choices (FMEA), but often the professional judgement of the team or querying a colleague with more experience at another institution are sufficient to make a choice.
D6: Implement and validate the permanent corrective action
After a PCA has been chosen, the performance of the PCA, using performance criteria specified in step D5, must be validated. Continuing with the example of insufficient assay sensitivity, a laboratory may choose to revalidate the assay using increased incubation time for the primary antibody. Depending on the application of the marker in question, achieving acceptable concordance with a pre-determined gold standard in the required number of cases for assay validation will constitute demonstration of effectiveness of the PCA.
After validation of the PCA, the team must develop a plan for implementation and clearly communicate this plan with all stakeholders. If the problematic assay was still being performed in-house while the process assessment was being performed, it may be necessary to consider or offer repeat testing on those patient samples with the implemented PCA.
D7: Prevent recurrence
After implementation, in order to prevent recurrence, regular, systematic monitoring to continually confirm effectiveness of the PCA is necessary. It may be the case that more frequent performance monitoring occurs in the short-term after implementation. After a period of time with acceptable performance, the laboratory may be reassured that PCA effectiveness is durable and shift to less frequent monitoring. However, if at any point monitoring indicates that the assay is not performing acceptably, a process improvement assessment may be re-initiated.
Additional changes in the laboratory at this stage include standardizing workflows, updating relevant policies, and sharing the process improvement assessment experience with others in the organization.
D8: Closure and team celebration
The last steps in the 8Ds framework include closure and celebration. In this step, process improvement assessment documents are archived and a team debrief occurs. It is recommended that document templates be used when possible to guide process improvement assessments. A team debrief is important to discuss the process improvement assessment process and to identify elements of conducting the assessment that may be improved next time. Lastly, it is crucial for team leaders to recognize the contributions of team members and celebrate the team’s success.
Resources
References
- Eight Disciplines of Problem Solving (8D). Quality one. 2021. Retrieved on February 16, 2021 from: https://quality-one.com/8d/#what
- VHA National Center for Patient Safety. 2021. Retrieved on February 16, 2021 from: https://www.patientsafety.va.gov/professionals/index.asp
- National Coordinating Council for Medication Error Reporting and Prevention. 2021. Retrieved on February 16, 2021 from: http://nccmerp.org/types-medication-errors
- Heher YK. A brief guide to root cause analysis. Cancer Cytopathol. 2017 Feb;125(2):79-82.
- Heher YK, Chen Y, VanderLaan PA. Pre-analytic error: A significant patient safety risk. Cancer Cytopathol. 2018 Aug;126 Suppl 8:738-744.
- Simon K. The Cause and Effect (A.K.A. Fishbone) Diagram. isixsigma.com. Retrieved on February 16, 2021 from: https://www.isixsigma.com/tools-templates/cause-effect/cause-and-effect-aka-fishbone-diagram/
- Cause Mapping ® Method Investigation File. Downloaded from www.thinkreliability.com. Template version 2020-v1.
The process improvement assessment will look different in the laboratory depending on the details of the situation and preferences of laboratory leadership. At minimum, a process improvement assessment requires a team approach (IHC laboratory director, technical specialist, lead technologist). It is recommended that the IHC laboratory develop a form or other tracking document to ensure actions such as those listed below are performed. Having the document as a working document in a shared drive is helpful to allow team members to update the document asynchronously.
Unacceptable responses [step D2] may arise in the pre-analytic, analytic, and/or post-analytic phases. Each of these must be queried in a standard, systematic way [step D4].
- Post-analytic
Double check the slide(s) and documentation (online and paper forms) to confirm that the reported result is the result that was intended to be submitted. - Analytic
- Technical: Review control slide to ensure the correct IHC antibody was run and performed appropriately. Discuss with lead IHC tech and technical specialist. Review in-house QA log. If the lab performs antibody dilutions, review the last 2-5 antibody dilution titer slides prepared upon receipt of new antibody lots to evaluate for a possible staining trend. If the lab uses an RTU preparation, review past lot-to-lot comparisons. Review results of prior IHC surveys. Review testing protocol to ensure no errors.
- IHC stain interpretation: Double check for possible pathologist (or in some instances image analysis) error.
- Biologic: No assay has perfect sensitivity and specificity. Antigens may be heterogeneously distributed in tissues.
- Pre-analytic
Double check that PT slides were handled appropriately in regards to storage and preparation prior to analysis. The CAP is generally responsible for guaranteeing absence of pre-analytic variables that would negatively affect a laboratory’s performance on an external quality assessment.
When clerical, pre-analytic, analytic, and post-analytic causes for an unacceptable response have been excluded, the possibility of a biologic explanation for the unacceptable response should be considered. Perhaps the clone has established sensitivity or specificity issues which may only be identified in proficiency challenges which are “low positive” (i.e., approach the average laboratory’s limit of detection). CAP IHC Survey Proficiency Summary Reports (PSRs) provide detailed data on clone-specific performance and will highlight platform-specific performance variation if identified in the survey. Similar information is also available from NordiQC assessments which are available online.
When examining an unacceptable result, it is also helpful to carefully review performance data for all tissue cores included in the survey. Re-review of the slides may identify a trend toward over- or under-calling staining quantity and/or staining intensity when compared to the results of other participating laboratories. Evaluating for possible trending will assist the lab in defining the scope and significance of the unacceptable result.
Process Improvement Assessment Examples
- Open all
- Close all
D0: Prepare and plan for the 8D process
The lab has intermittent, unacceptable responses on ER proficiency testing, usually in cases near the 1% positive quantitative threshold. More often, the ER is resulted as negative when the intended response is low positive, but occasionally, ER is resulted as low positive when the intended response is negative. Similarly, in daily case work, there is often intraobserver variability among pathologists regarding ER interpretation. Based on annual monitoring data, the percent of ER negative breast cancers observed in the laboratory is within published benchmarks (<25-30%). It is anticipated that the issue is possibly multi-factorial, including pathologist read-out error and/or suboptimal assay conditions (either over- or under-staining).
D1: Form a team
Representatives from stakeholder groups including lab staff/supervisor, medical director, breast pathologists and other pathologists resulting ER IHC, breast oncologists
D2: Describe the problem
The lab is experiencing unacceptable responses in ER proficiency testing. In most instances, a clear trend in the unacceptable responses is not appreciated and pathologists routinely disagree on quantitation.
D3: Interim containment action
Due to ER’s status as a highly utilized predictive marker with significant impact on patient care, it would seem prudent to temporarily suspend in-house testing and prioritize time and resources for this process improvement assessment so that a conclusion is reached in less than 10 business days. However, if the delay in TAT due to send-out is unacceptable, in-house testing could be performed with temporary send-out confirmatory testing for any ER low positive or ER negative case (with billing charges removed for the in-house test if send-out is needed).
D4: Root cause analysis (RCA)
- Post-analytic
The TMAs are re-reviewed by a blinded pathologist who did not participate in the initial proficiency test review. Significant disagreement is observed in cases in question. - Analytic
If unacceptable responses fail to show a consistent trend, or if there is not a known source of random variation in the laboratory, then this suggests that analytic problems do not wholly explain the observed problem. However, if the majority of the intended responses trend in one direction, this may indicate that some degree of assay re-optimization would help the situation. After review of the PSR, assay conditions are similar, but not identical, to the majority of laboratories using the same clone/platform. - Pre-analytic
PT slides were handled according to directions upon arrival. No pre-analytic variables were felt to contribute to the problem.
D5: Permanent corrective action (PCA)
In order to determine the PCA, a Pareto diagram was created.
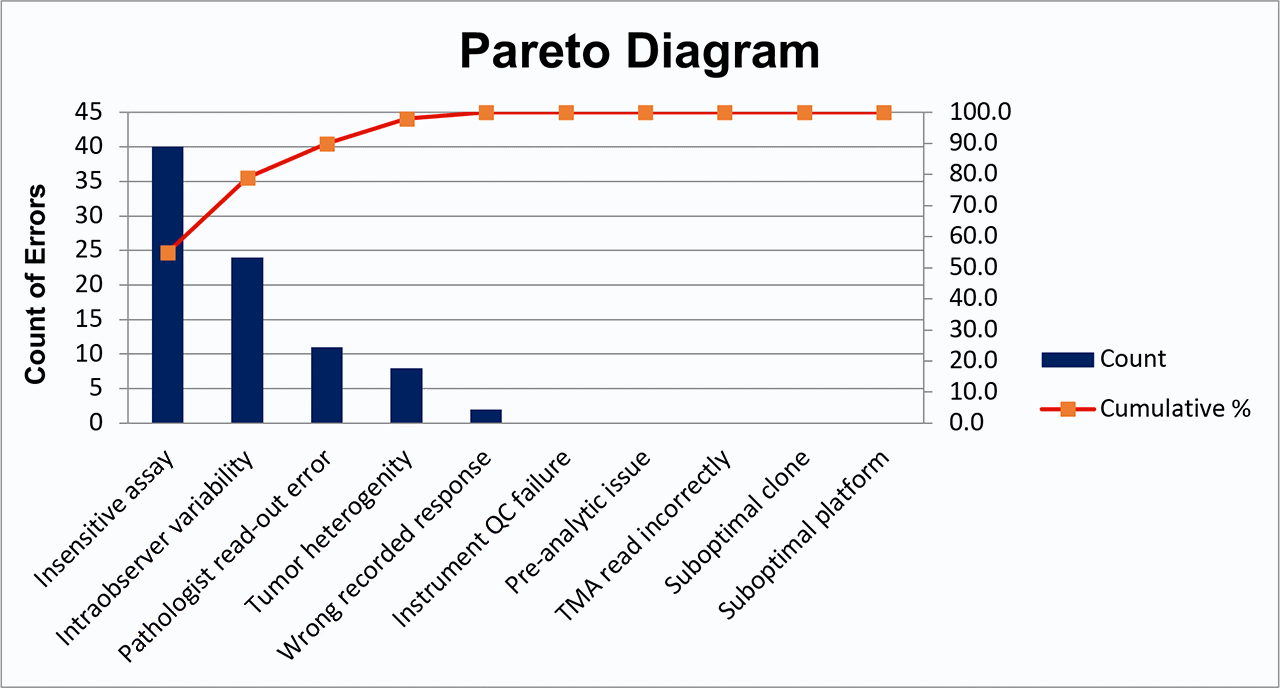
A Pareto diagram is a visual representation of the percent of error assigned to each possible cause. A vertical line is dropped from 80% of the cumulative percent curve to the x-axis. Possible causes to the left of this vertical line account for 80% of the observed error and are considered most important to include in PCA. Possible causes to the right of this vertical line account for fewer than 20% of the observed error and are considered less important at this time.
After review of the Pareto diagram, it is determined that the PCA will be two-fold. To address analytic concerns, the assay will be re-validated according to existing recommendations for ER validations to align assay conditions more closely with those of laboratories using similar clone/platform. To address pathologist intraobserver variability and read-out error, the laboratory will consider digital image analysis.
All pathologists will also be reminded of the 2020 ASCO/CAP ER/PR guideline updates and the instituted laboratory policy for prospective adjudication of ER low positive and ER negative cases. For example, an internal policy is implemented in which any case within or approaching the 1-10% low positive category is shown to a second pathologist before reporting, with any discordance reconciled by a third pathologist.
D6: Implement and validate the permanent corrective action
The revalidated assay will be implemented, and pathologists appropriately use adjudication procedure.
D7: Prevent recurrence
Continued participation in PT. Attention to ER performance monitoring reports. Consider adding ER low positive data to ongoing quality monitoring to observe trends. Could consider random sampling of reported ER low positive and ER negative cases for re-review for group educational purposes.
D8: Closure and team celebration
D0: Prepare and plan for the 8D process
The PR PT failure occurred in the first proficiency test event after the PR assay was re-validated due to clinician generated concern that the observed rate of ER negative/PR positive breast cancer was too high in the patient population. Initially anticipated significant time requirement from the lab medical director and laboratory staff to perform revalidation and repeat testing of patient samples since the re-validated protocol was launched.
D1: Form a team
Laboratory medical director, laboratory supervisor, laboratory tech staff, chief of pathology at sites with PT failure, representative breast oncologist (who participated in the initial re-validation).
D2: Describe the problem
Failure to achieve acceptable (90%) concordance with intended responses on a graded proficiency test.
D3: Interim containment action
Initial examination of the unacceptable responses indicated consistent trend toward false negative results. Since the concern that prompted the initial revalidation was concern about false positive PR results, it was felt that the potential for false negative would have insignificant impact on immediate patient care. Therefore, testing was allowed to continue in-house for the duration of the process improvement assessment (PIA), pathologists and breast oncologists were notified, and plans were made to perform repeat testing on all PR negative cases resulted between launch of the prior re-validated assay and re-launch of the assay when the corrective action identified by the current assessment was implemented.
D4: Root cause analysis (RCA)
- Post-analytic
The TMA is re-reviewed and submitted responses confirmed to reflect staining on the slide (no clerical errors in response submission). - Analytic
- Assay conditions: Due to the prior assay changes to mitigate clinician concern regarding false positive PR results, the primary antibody incubation time had been recently reduced. In the PIA for that re-validation, a preventive action plan stipulated that if a high rate of potential false negatives were observed, the assay conditions would be further adjusted by making a small increase in primary antibody incubation time, which would align with the manufacturers recommendations and the majority of laboratories using the same clone (per the CAP PSR). Antigen retrieval conditions were already aligned with those of other laboratories using the same clone/platform.
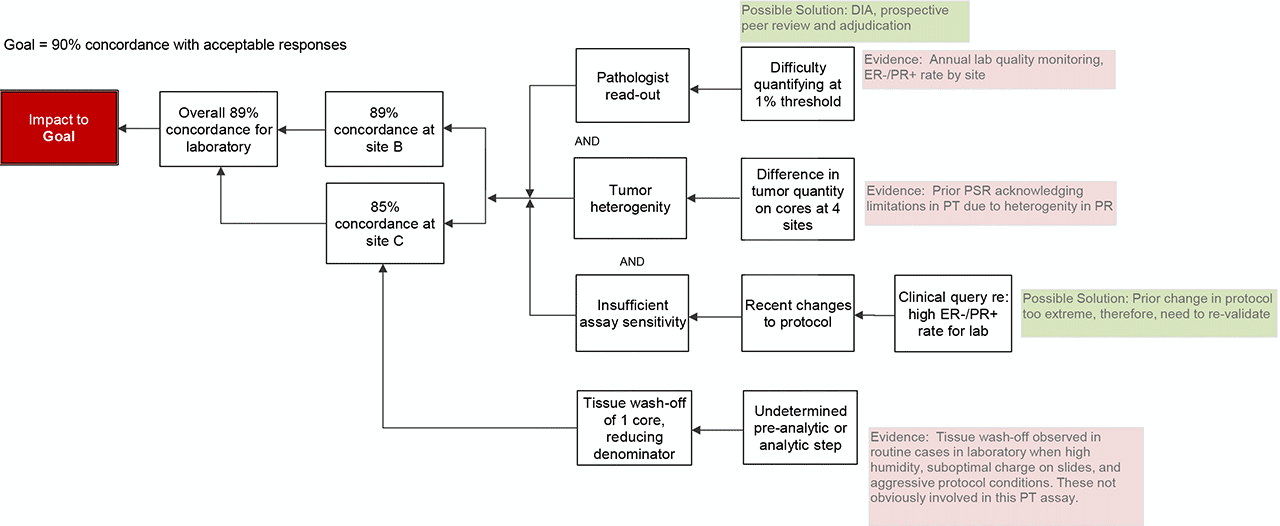
- Pathologist read-out: In review of the unacceptable cores, laboratory quarterly monitoring reports for breast predictive markers, and daily cases, it appeared that pathologists were having 2 issues: 1) difficulty with reproducible quantification at the 1% positive threshold and 2) dismissing weak, nuclear staining as non-specific.
- Biology: Heterogeneity of tumor quantity is a well-established factor that effects standardization in TMA based surveys. The lab in question prepares PT materials for interpretation at 4 CLIA licensed sites. By comparing the 4 TMAs after the fact, it was confirmed that there was reasonably consistent staining intensity across the interpreted TMAs, but there was significant variability in the quantity of tumor in slides (affecting denominator and subsequently % positive calculation).
- Pre-analytic
One site observed complete tissue wash-off of 1 core. Therefore, only 19 responses could be provided and the denominator for calculating concordance rate was reduced. Had this tissue remained on the slide and reported result was concordant with the intended response, this site would not have achieved <90% concordance. Some degree of tissue wash-off is observed in routine clinical cases in the laboratory. Past PIAs to address this issue specifically have identified high humidity conditions, insufficient or loss of charge of glass slides, and extended or aggressive protocols as causes of tissue wash-off. However, it is not anticipated that these factors contributed significantly in this case due to the controlled pre-analytic conditions of PT materials and not overly aggressive assay conditions. Therefore, the cause of this tissue wash-off remains uncertain.
- Conclusion
The root cause is likely multifactorial including both analytic assay concerns and pathologist read-out concerns.
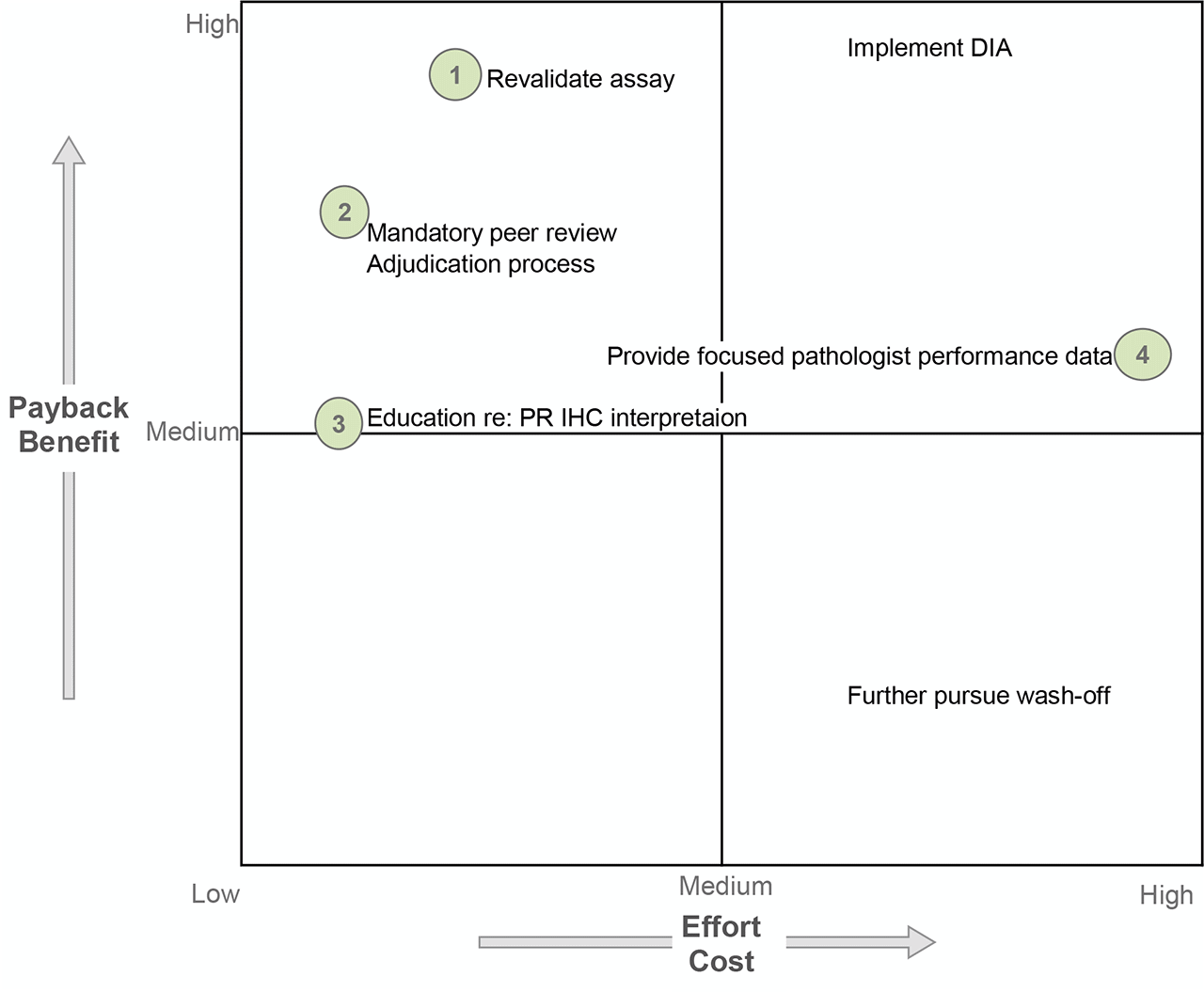
D5: Permanent corrective action (PCA)
Several possible solutions exist to address the assay concerns and pathologist read-out concerns. The time/cost requirements to complete assay revalidation was deemed necessary to produce an assay with acceptable performance so as to continue performing the test in-house (PR is no longer monitored). A mandatory prospective peer review was initiated for all PR negative and PR low positive cases; however, digital image analysis (DIA) was not further pursued due to high cost and implementation requirements. Pathologist education was performed due to anticipated low time/energy cost but, admittedly, of uncertain yield other than increasing awareness of the need to be conscientious at the 1% threshold and seek other opinions. Finally, site-specific retrospective ER-/PR+ breast cancer data were generated and shared for focused performance evaluation; however, a formal adjudication procedure was not ever defined or implemented.
D6: Implement and validate the permanent corrective action
Primary antibody incubation duration was increased 4 minutes to align with manufacturer recommendations and the conditions reported by the majority of laboratories using the same clone. A full assay revalidation was performed. The launch of the new assay was announced to breast oncologists. All patient samples with PR negative results since the last assay change were re-tested with the new assay conditions at no charge to the patient.
D7: Prevent recurrence
Breast predictive marker quality monitoring was expanded to include site-specific data for ER-/PR+ breast cancer. As a result of cumulative assay changes, a compensatory increase in triple negative and ER+/PR- breast cancer was anticipated, and these metrics were included accordingly. The laboratory continues to participate in proficiency testing. The internal process for annual pathologist competency assessment, as required for breast predictive markers, was to be re-evaluated.
D8: Closure and team celebration
Monitoring of site-specific ER-/PR+ breast cancer was planned to continue for 12 months. If at that time, the rate of ER-/PR+ breast cancer was stable at <2% and there were no clinician concerns, the corrective action plan would be closed. If not, the lab would re-evaluate.
D0: Prepare and plan for the 8D process:
The lab achieves unacceptable concordance with intended responses on ALK proficiency testing. Initially anticipate an analytic issue with the assay and allocate several hours of lab tech and lab director time to troubleshoot the assay.
D1: Form a team
Lab tech/lab supervisor, medical director.
D2: Describe the problem:
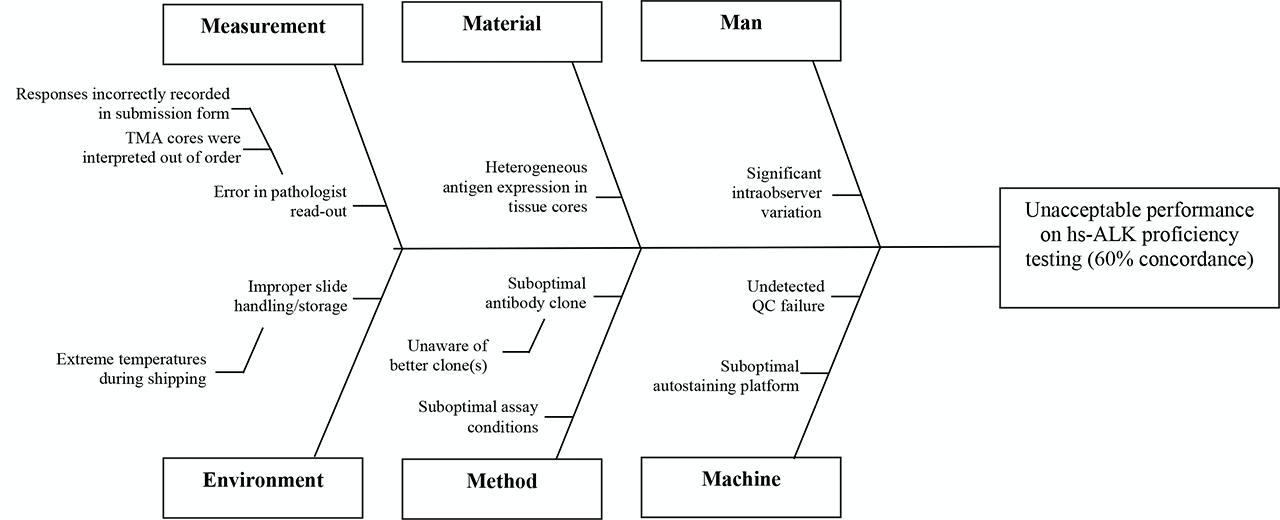
The lab achieved acceptable results on 6 of 10 cores. The lab registered unacceptable results on 4 of 10 cores – in all unacceptable cores, the intended response was positive and the lab’s submitted response was negative. This suggested insufficient assay sensitivity. A team member suggests creating a Fishbone diagram to consider whether there may be alternative or additional causes of the unacceptable PT performance.
D3: Interim containment action
Due to high rate of false negative results, and that a negative result has the significant effect of excluding a patient from receiving therapy, the lab will temporarily cease in-house predictive ALK IHC and perform as a send-out.
D4: Root cause analysis (RCA)
Post-analytic
The TMA is re-reviewed and submitted responses confirmed to reflect staining on the slide (no clerical errors)
Analytic
The PSR from the past ALK survey is reviewed for comparison of assay parameters with other laboratories – it is noted that the majority of labs use highly sensitive ALK clones. And that other laboratories observing negative results on the 4 cores in question in this analysis were predominately also using ALK1 (not a highly sensitive ALK clone).
Pre-analytic
PT slides were handled according to directions upon arrival. No pre-analytic variables felt to contribute to the problem.
Conclusion
The root cause of the problem is use of an insufficiently sensitive clone.
D5: Permanent corrective action (PCA)
Based on additional literature review, comparison with other laboratories via the PSR, and review of recommendations to perform predictive ALK testing using highly sensitive clones, the lab will change to a highly sensitive ALK clone. Alternatively, re-optimization of the assay using ALK1 was considered; however, available literature suggests that assay parameters have not been identified for ALK1 that produce acceptable concordance with ALK rearrangement.
D6: Implement and validate the permanent corrective action
New clone requires full revalidation using 20 positive and 20 negative cases. The comparator method will be results of ALK FISH and/or molecular. Clinicians, especially pulmonary oncologists, will be notified of the RCA and offered the opportunity to perform repeat testing using the highly sensitive clone at no cost to patient.
D7: Prevent recurrence
ALK1 is felt to still be a diagnostically relevant immunostain that should be retained on the test menu. There is potential for confusion and inappropriate ordering if there are two “ALK stains” in the IHC menu. The order for highly sensitive ALK will be specified by clone name (HSALK). Periodic monitoring of highly sensitive ALK results will be performed to confirm that ~5% of lung cancers are positive by highly sensitive ALK immunohistochemistry. Automated reminder will be set-up to prompt at least annual literature review regarding the availability and performance of new highly sensitive ALK clones.
D8: Closure and team celebration
Additional comments:
The IHC committee has observed in past highly sensitive ALK proficiency testing, up to 15% of participants continue to use ALK1 instead of highly-sensitive ALK clones.
D0: Prepare and plan for the 8D process
The lab has intermittent, unacceptable responses on BRAF V600E proficiency testing. Unacceptable responses are usually cases where the intended response was positive and the submitted response was negative. Anticipate missing low positive cases requiring assay re-optimization and revalidation. Anticipate allocating several hours of lab staff and medical director time for process improvement assessment and resolution.
D1: Form a team
Lab tech/supervisor, medical director, possibly staff in molecular genetics who can provide confirmed V600E mutation cases.
D2: Describe the problem
Over the last several rounds of BRAF V600E PT, the lab has had intermittent false negative results, indicating insufficient assay sensitivity.
D3: Interim containment action
Although a problem requiring resolution, the frequency of false negative results seems low level. The interim plan will be to continue in-house testing but perform confirmatory molecular analysis for all BRAF V600E IHC negative results.
D4: Root cause analysis (RCA)
- Post-analytic:
The TMA is re-reviewed by pathologists most experienced at interpretation of BRAF V600E IHC in the group and submitted responses confirmed to reflect staining on the slide (no clerical errors and interpreted correctly) - Analytic
The PSR from past BRAF V600E surveys is reviewed for comparison of assay parameters with other laboratories – it is noted that the majority of labs using the same clone/platform use a longer primary antibody incubation duration and more aggressive antigen retrieval. Past lot-to-lot comparisons are retrieved and reviewed – no decrement in staining observed over time. The original BRAF V600E validation documentation is retrieved and reviewed showing strongly positive staining in all positive cases. On-slide positive control tissue selected from the positive validation cases is strongly positive. - Pre-analytic
PT slides were handled according to directions upon arrival. No pre-analytic variables felt to contribute to the problem. - Conclusion
The root cause of the problem is likely suboptimal assay conditions. Absence of low positive cases from the validation cohort and on-slide control tissue likely contributed to a suboptimal initial validation.
D5: Permanent corrective action (PCA)
Re-optimize and revalidate the assay.
D6: Implement and validate the permanent corrective action
Assay to be revalidated using longer antibody incubation duration (or other parameters). A larger number of cases will be included in the validation cohort to characterize the spectrum of positivity in cases, including low positivity cases. A low positive case will be identified and used as the on-slide positive control tissue.
D7: Prevent recurrence
Continued participation in PT and attention to fluctuations in the low positive control. Could consider molecular testing of a random sample of IHC negative cases, to confirm no recurrent issue with false negatives.
D8: Closure and team celebration
Additional comments:
Review of CAP PT survey data for BRAF V600E collected in recent years indicates that most “unacceptable” results occurred in assessment of BRAF V600E status in colonic adenocarcinoma samples. It has been speculated that a lower level of mutant protein expression in these tumors compared to others such as melanoma may be the underlying issue. If a lab used only melanoma tissue in the assay validation process, it may select a staining condition that is optimized for detecting abundant mutant protein in melanoma, which may be insufficiently sensitive for reliable detection of mutant protein in colonic adenocarcinoma. Therefore, validation of the staining protocol has to be performed using all tumor types for the intended clinical applications. Correct interpretation of staining results may also be challenging for some colonic adenocarcinoma samples and orthogonal testing methods should be considered in challenging cases.
D0: Prepare and plan for the 8D process
The lab has experienced intermittent, unacceptable responses on KIT proficiency testing. The majority of the unacceptable responses occurred where the intended response was negative and the submitted response is positive. Appropriate KIT staining is localized to the cytoplasm. The majority of the unacceptable responses demonstrated nuclear staining. Based on this preliminary review of the data, the lab leadership anticipates the cause of nuclear staining is due to extended or overly aggressive assay conditions. Lab leadership anticipates allocating several hours of lab staff and medical director time for process improvement assessment and resolution.
D1: Form a team
Lab staff/supervisor, medical director
D2: Describe the problem:
The lab is experiencing unacceptable responses in KIT proficiency testing. In most instances, the intended result is negative and the lab has submitted a result of positive, indicating insufficient specificity.
D3: Interim containment action
Although KIT is a diagnostically useful marker in some situations, KIT serves a limited role as a predictive marker. Diagnostically, there are alternative markers to KIT testing available in the laboratory (DOG1 in GIST; CD34 or MPO in AML), as such there is limited potential for negative adverse effect on patient care. Will notify pathologists of the concern for potential over-staining and temporarily recommend against use of the in-house stain while process improvement assessment is on-going.
D4: Root cause analysis (RCA)
- Post-analytic
The TMA is re-reviewed and submitted responses confirmed to reflect staining on the slide (no clerical errors) - Analytic
The PSR from the past KIT survey is reviewed for comparison of assay parameters with other laboratories – it is noted that the majority of labs use assay parameters that are less aggressive or shorter duration than what is currently used in the laboratory. - Pre-analytic
PT slides were handled according to directions upon arrival. No pre-analytic variables felt to contribute to the problem. - Conclusion
The root cause of the problem is likely overly aggressive or extended assay conditions.
D5: Permanent corrective action (PCA)
Assay to be reoptimized considering using shorter antibody incubation duration, less aggressive antigen retrieval conditions, omitting additional heat options, etc. Conditions will be titrated until nuclear staining is not observed.
D6: Implement and validate the permanent corrective action
The reoptimized and revalidated protocol will be implemented. At that time, pathologists will be notified of the change in assay parameters and the recommendation against performing in-house testing will end.
D7: Prevent recurrence
Continued participation in PT and attention to fluctuations in control tissue. Return of nuclear staining would require another process improvement assessment.
D8: Closure and team celebration