- Home
- Member Resources
- Articles
- What’s new in AML Classification (WHO 2022 vs International Consensus Classification)
Diagnosis and classification of acute myeloid leukemia (AML) have significantly changed in 2022 with the newly proposed WHO 5th edition and International Consensus Classification (ICC) classification of hematopoietic neoplasms (subsequently referred to as WHO 2022 and ICC 2022 classification, respectively).1,2 Both systems emphasize integration of clinical, molecular/genetic, morphologic and immunophenotypic parameters to provide evidence-based classification of AML, and to facilitate precision diagnosis and prognostication, improve treatment, and allow the design of innovative clinical trials for AML. The goal of this article is to summarize the changes from the previous WHO classification revised 4th edition (aka WHO 2017), and to highlight the differences between these new classifications (download Table 1. AML Classification Systems in 2022 [Excel]) and the impact on pathology practice, particularly in terms of molecular hematopathology.
The most notable change is the blast count used to define AML. In WHO 2017, the diagnosis of AML is defined as a blast count of greater than or equal to 20% in the bone marrow and/or blood. Exceptions are acute promyelocytic leukemia (APL) and core binding factor AML (i.e., RUNX1-RUNX1T1, CBFB-MYH11), in which AML can be diagnosed with a blast count of less than 20% without a specific cutoff value of blast percentage.
WHO 2022 now eliminates 20% blast requirement for AML diagnosis for all AML with defining genetic abnormalities listed in Table 1 (with the exception of AML with BCR::ABL1 fusion, AML with CEBPA mutation, and other/rare defined genetic alterations which still require 20% blasts ). This change is based on studies demonstrating that patients with any of these abnormalities and <20% blasts (defined as myelodysplastic syndrome, [MDS] per WHO 2017) have clinical outcomes that resemble those with higher blast counts. For example, cases previously classified as MDS or MDS/MPN with NPM1 progress to AML in a short period of time.2
In contrast to WHO 2022, ICC 2022 requires blast cutoff of 10% for AML with recurrent genetic abnormalities listed in Table 1 (with the exception of AML with BCR::ABL1 fusion, still requiring 20% blasts). Both systems indicate that the requirement of 20% blasts for AML with BCR::ABL1 fusion is to avoid overlap with chronic myeloid leukemia (CML).
The defining genetic abnormalities for AML in WHO 2022 and the recurrent genetic abnormalities ICC 2022 are largely the same as WHO 2017 with mild differences shown in Table 1. AML Classification Systems in 2022 [Excel]. For example, in cases with CEBPA mutations, WHO 2022 includes biallelic (biCEBPA) and single mutations located in the basic leucine zipper (bZIP) region of the gene (smbZIP-CEBPA) while ICC 2022 only includes in-frame bZIP CEBPA mutations. The changes are made due to the favorable prognosis associated with CEBPA mutation is predominantly associated with in-frame bZIP CEBPA mutations based on recent studies.1 Of note, both eliminate the provisional entity of AML with mutated RUNX1 in WHO 2017 due to lack of data to support this as a unique entity. The AML with mutated RUNX1
cases are now classified based on criteria/approaches listed in Table 1
and Figure 1.
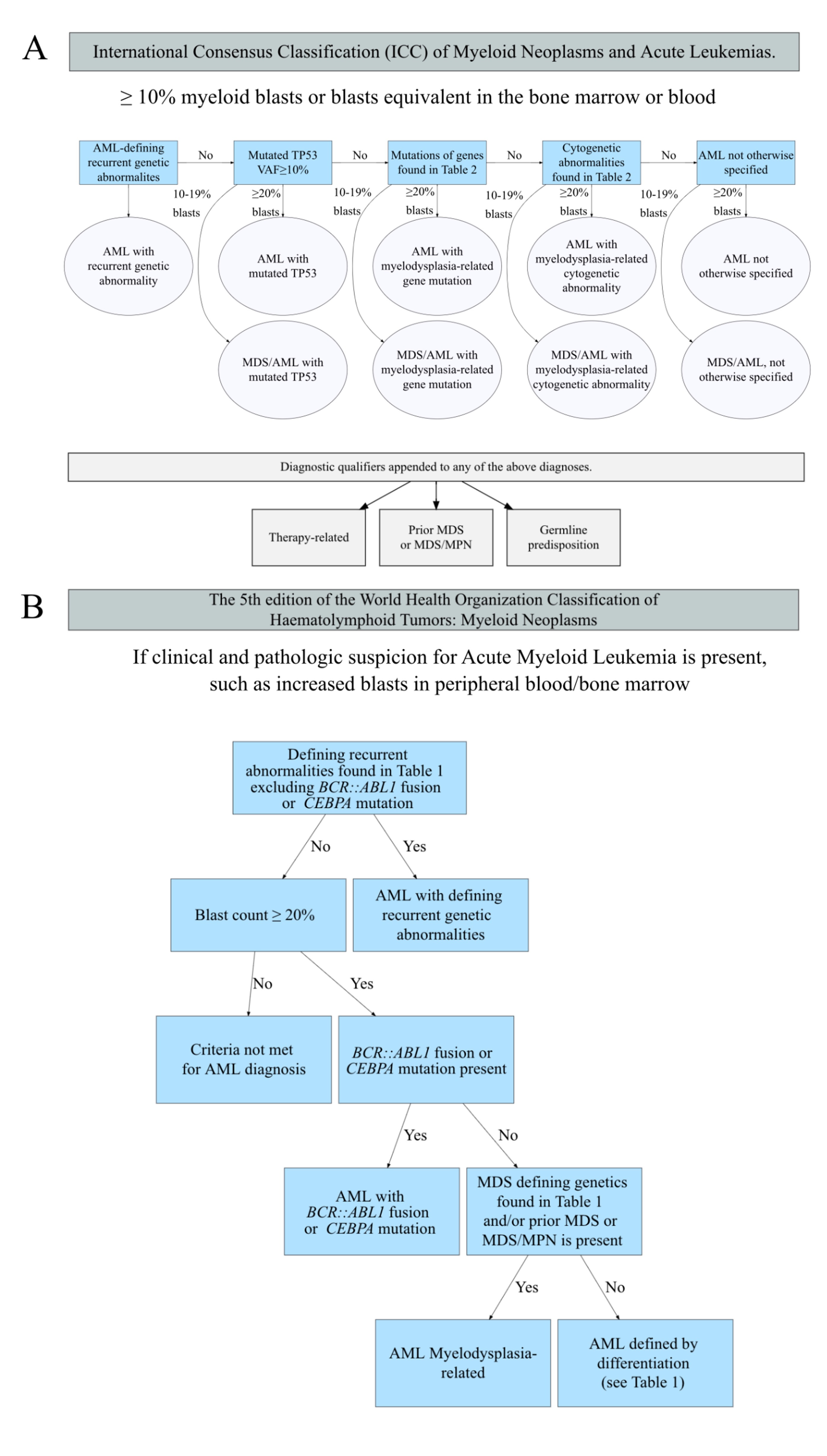
Several changes are made to the WHO 2017 entity AML with myelodysplasia-related changes (AML-MRC). This is now called AML, myelodysplasia-related (AML-MR) in WHO 2022, and separated into two categories in ICC 2022 (download Table 2. Cytogenetics and molecular abnormalities defining AML, myelodyslaspia-reltaed, WHO 2017/2022 and ICC 2022 [Excel]): AML with myelodysplasia-related gene mutations and AML with myelodysplasia-related cytogenetic abnormalities. Both classifications require ≥20% myeloblasts for this category of AML and remove morphology alone as a diagnostic premise. AML-MR include cases with defined genetic abnormalities listed in Table 2 and / or with a prior history of MDS or MDS/MPN (myeloproliferative neoplasm). In ICC 2022, a history of MDS or MDS/MPN is considered a diagnosis qualifier but not a classifier (i.e., including the history as a descriptive condition in the diagnosis but not as a classification criterium). Of note, both expand the genetic abnormalities to include mutations in specific genes (with minor differences between the two highlighted in red in Table 2 beyond karyotyping abnormalities in WHO 2017. Additionally, several balanced translocations used to define AML-MRC in WHO 2017 are not included in either 2022 classification.
In ICC, a new major category of AML, AML with mutated TP53 is created. This category requires at least 20% of blasts and any somatic TP53 mutation at a variant allele fraction of greater than 10%. This group supersedes the classification of AML with myelodysplasia-related gene mutations/cytogenetic abnormalities (Figure 1). This is created due to the very poor prognosis of the patients and likely unique biology (e.g., often associated with complex karyotype abnormalities) associated with TP53 mutations. Of note, cases with 10 to 19% blasts are classified as MDS/AML with mutated TP53 (see Figure 1 and below).
The remaining cases with blast counts >20% are now classified as AML, defined by differentiation by WHO and remain AML, not otherwise specified (NOS) by ICC; both are based on the same definitions of AML, NOS from WHO 2017. However, as the vast majority of pure erythroid leukemia (PEL) cases are associated with TP53 mutation, most of these cases will now be classified as AML with mutated TP53 in the ICC. Additionally, in WHO 2022, the diagnosis of AEL (acute erythroid leukemia, a new name for PEL) supersedes AML-MR. Diagnostic criteria for AEL are erythroid predominance, typically ≥80% of bone marrow elements, of which ≥30% are proerythroblasts (or pronormoblasts).
In ICC, a new category MDS/AML (eliminating the MDS with excess blasts, grade 2 in WHO 2017) has been created for cases with 10 to 19% of blasts in peripheral blood and / or bone marrow. This is to allow patients in this group to be able to participate in MDS and AML clinical trials depending on clinical conditions. The diagnostic criteria for this category are identical to those of AML requiring over or equal to 20% blasts (Figure 1). In contrast, WHO 2022 continues to classify these patients as MDS-IB (increased blasts, term changed from excess blasts) grade 2 to avoid over-treatment. However, WHO indicates that MDS-IB2 can be considered as AML for therapeutic purposes if clinically indicated.
In WHO 2022, myeloid neoplasms that arise secondary to exposure to cytotoxic therapy or germline predisposition are classified into the major category of myeloid neoplasms, secondary, with 3 sub-categories: myeloid neoplasm post cytotoxic therapy, myeloid neoplasms with associated germline predisposition and myeloid proliferation associated with Down syndrome. While, in ICC, these conditions are considered as diagnostic qualifiers rather than classifiers (Figure 1).
Figure 1 shows the schemes of classifying AML using ICC and WHO 2022. The following example shows how to classify the AML patients in practice. A patient with a history of soft tissue sarcoma post-chemotherapy developed MDS and then progressed to AML with 21% blasts, TP53 mutation (variant allele fraction of 12%), and complex karyotype. Per ICC 2002 this patient’s diagnosis is AML with mutated TP53, therapy-related, progressed from MDS. The WHO 2022 classification is: myeloid neoplasm (AML progressed from MDS), post cytotoxic therapy (MN-pCT).
Additionally, the ELN (European LeukemiaNet, https://www.leukemia-net.org, 220 participating centers in 44 countries across Europe focusing on studying leukemias) also updated their 2017 genetic risk stratification of AML (aka ELN 2017) this summer to be in line with ICC classification (ELN 2022).3,4 Notable changes are described below and in Table 3. ELN 2022 risk classification by genetics at initial diagnosis [Excel] The FLT3-ITD allelic ratio is no longer considered in the risk classification; therefore, AML with FLT3-ITD (without adverse-risk genetic lesions) are now categorized in the intermediate-risk group, regardless of the allelic ratio or presence of NPM1 mutation. The reason for this change is due to the difficulties of standardizing the measurement the FLT3-ITD
allelic ratio by different assays, the modifying impact of midostaurin-based therapy on FLT3-ITD, and the increasing role of minimal/measurable residual diseases in treatment decision.3 The mutated genes associated with adverse prognosis are expanded to include TP53, ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2
genes. Finally, in line with ICC classification in-frame mutations affecting the bZIP region of CEBPA
are now categorized in the favorable-risk group replacing biallelic CEBPA mutations.
With the above changes, appropriate diagnosis, classification, and risk stratification of AML cases (Figure 1 and Table 1, Table 2, and Table 3) will require integration of morphology, clinical history and study results of cytogenetics, fluorescent in situ hybridization (FISH) and / or next generation sequencing (NGS) of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) targeting the genetic abnormalities. Testing approaches should consider that some important rearrangements (e.g., NUP98, KMT2A, MECOM) may be cryptic on conventional karyotyping. Additionally, ELN 2022 also indicates the importance of detecting measurable/minimal residual diseases by flow cytometry and/ or molecular/NGS testing after initial treatment in determining the prognosis and follow-up treatment options including stem cell transplants for AML patients3,5. Consequently, it is likely that the volume of NGS testing, which is the most effective way of detecting these genetic abnormalities, and flow cytometry testing will significantly increase over the next few years with the need to practice these new classification schemes and to provide precision treatment for the patients. The pathology communities should be aware of these changes and prepare to handle the increased testing volumes.
References
- D Arber, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data, Blood 2022;140(11):1200-1228. doi:10.1182/blood.2022015850. CC BY-NC-ND 4.0.
- JD Khoury, et al The 5th edition of the World Health Organization Classification of Hematolymphoid Tumors: Myeloid and Histiocytic/Dendritic Neoplasms, Leukemia 2022;36(7):1703-1719. doi:10.1038/s41375-022-01613-1. CCBY 4.0.
- H Dohner, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN, Blood 2022;140(12):1345-1377. doi:10.1182/blood.2022016867. CC BY-NC-ND 4.0.
- H Dohner, et al. Diagnosis and management of AML in adults: 2017 recommendations from an international expert panel on behalf of the ELN, Blood 2017;129: 424-47.
- M Heuser, et al. 2021 Update on MRD in acute myeloid leukemia: A Consensus document from the European LeukemiaNet MRD Working Party, Blood 2021;138(26):2753–2767

Chung-Che (Jeff) Chang, MD, PhD, FCAP, is the medical director of Hematology and Molecular/Genomic Laboratory at AdventHealth-Orlando, and professor of pathology, University of Central Florida. He currently serves as associate editor for Archives of Pathology and Laboratory Medicine, and a member of the Personalized Healthcare Committee for the College of American Pathologists (CAP). He was the principal investigator of several National Institutes of Health (NIH)/National Cancer Institute (NCI) grants to study myeloma, myelodysplastic syndromes, and lymphoma. Dr. Chang’s research interests include the use of next-generation sequencing technologies for clinical diagnostics and biomarker discovery.

Amirah Kuzu, MD, is currently a fourth year Pathology Resident at Orlando Health. She will be continuing her training as a Hematopathology Fellow at the University of New Mexico in the following year. Dr. Kuzu’s research interests include personalized medicine, myeloproliferative neoplasms and coagulation.