- Home
- Member Resources
- Articles
- Top 5 Takeaways on FDA LDT Risk Classification
The College of American Pathologists' (CAP) recent webinar, Understanding the Impact of the FDA's LDT Risk Classification on Your Laboratory, discussed the differences between FDA and CLIA classifications, requirements under the FDA risk categories, and activities included in the FDA's general and special controls, as well as examples of common tests and how the FDA requirements will apply to them. Featuring CAP President Donald S. Karcher, MD, FCAP, and Timothy Stenzel, MD, former director of the FDA's Office of In Vitro Diagnostics and current CEO of Grey Haven LLC, this was the first webinar in the six-part series Understand and Prepare for the Impact of the FDA’s LDT Final Rule.
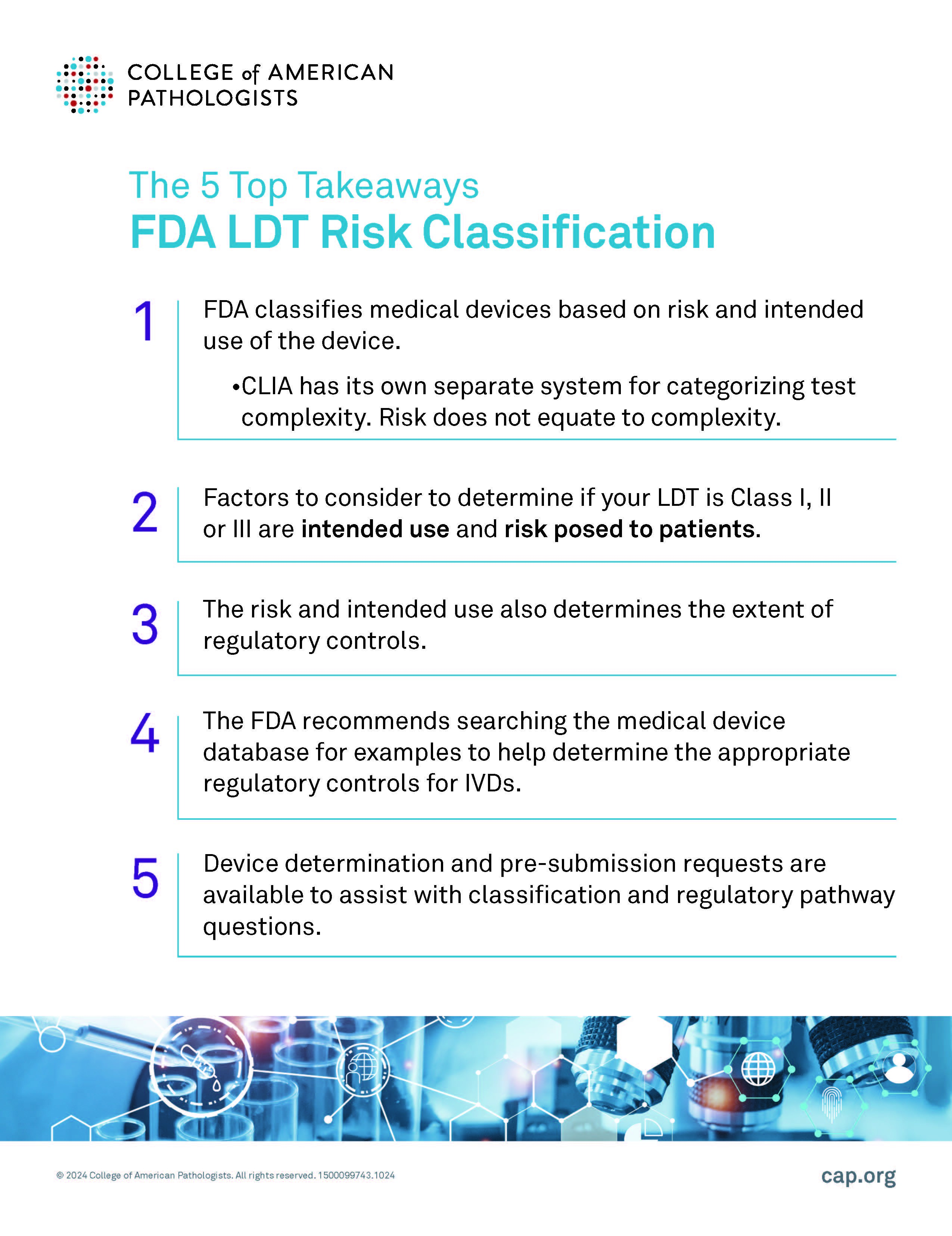
Here are five key takeaways from the webinar:
Risk does not equate to test complexity.
When it comes to regulating medical devices, the U.S. Food and Drug Administration (FDA) classifies them based on two critical factors: the device's intended use and the risk it poses to patients. However, it's important to note that the Clinical Laboratory Improvement Amendments (CLIA) operates under a different framework, using its own system to categorize test complexity. While both the FDA and CLIA play vital roles in ensuring patient safety, the distinction between risk and complexity is key: risk does not necessarily equate to test complexity.
The FDA uses a risk-based classification system for medical devices including LDTs and IVDs.
Medical devices and tests are categorized into Class I, Class II, or Class III according to the level of regulatory control (mitigations) that are deemed necessary by the FDA to ensure accurate results.
Test risk and intended use also determine the extent of regulatory controls.
The primary factors influencing the classification of a device are its intended use and the risk it may pose to patient health.
- Class I: These are low-risk with minimal risk for harm.
- Class II: Moderate-risk tests/devices that fall in a mid-range risk for harm.
- Class III: These are high-risk tests that support or sustain human life, which presents a potential for unreasonable risk of harm.
For example, if a device's failure could lead to serious harm, it will likely fall into Class III, while devices with a low risk of adverse outcomes are classified in Class I.
The FDA recommends searching the medical device database to help determine the appropriate regulatory controls.
For laboratories developing diagnostic tools, understanding whether an LDT is considered a Class I, II, or III device is crucial for regulatory compliance. The FDA suggests using its medical device database to review similar products, which can offer insights into the appropriate classification and associated regulatory controls. This may also prove helpful in understanding what specific IVDs fall within a given device type and how such IVDs are regulated.
Device determination and pre-submission requests are available.
To assist with classification and regulatory questions, the FDA also provides tools like device determination and pre-submission requests, which are valuable resources for manufacturers navigating the regulatory pathway.
While both the FDA and CLIA aim to protect patient safety, they focus on different aspects of regulation—risk and complexity, respectively. Ensuring that your LDT is correctly classified is a critical step in aligning with FDA regulations and ensuring patient safety. To learn more about FDA risk classification, watch the webinar recording and download the presentation.
To continue preparing for the FDA LDT final rule, register for the upcoming webinar, Ready Your Laboratory for the FDA's Stage 1: Adverse Event Reporting Requirement, on November 7, 2024, at 12 PM Central Time. Bobbi S. Pritt, MD, FCAP, chair, Council on Scientific Affairs, and Earle S. Collum, MD, FCAP, vice chair, Council on Accreditation will discuss existing accreditation requirements and ways laboratories can comply with the FDA rules on medical device-related adverse event reporting. They will review best practices that relate to quality management and review real-world scenarios.