- Home
- Member Resources
- Articles
- Pathologists at the Forefront: Shaping the Future of Cancer Treatment with Molecular Tumor Boards
Molecular Tumor Boards
What is a mTB?
The goal of personalized healthcare (PHC) is to customize treatment to the individual characteristics of each patient, maximizing efficacy and minimizing adverse effects. This paradigm considers genetic, environmental, and lifestyle factors. Central to PHC is embracing new validated technologies like circulating tumor DNA (ctDNA), digital health, and next-generation sequencing (NGS), while incorporating the evolution of existing ones – for example, multiplexed histology.1 NGS provides detailed genetic information, uncovering mutations and variations linked to diseases, leading to deeper more comprehensive understanding of pathogenesis and opportunities for novel targeted therapies. However, NGS results can be challenging to interpret in the context of technical quality, biologic relevance, and clinical impact, leading to the use of molecular tumor boards (mTBs) to support interpretation and align across functional expertise.2
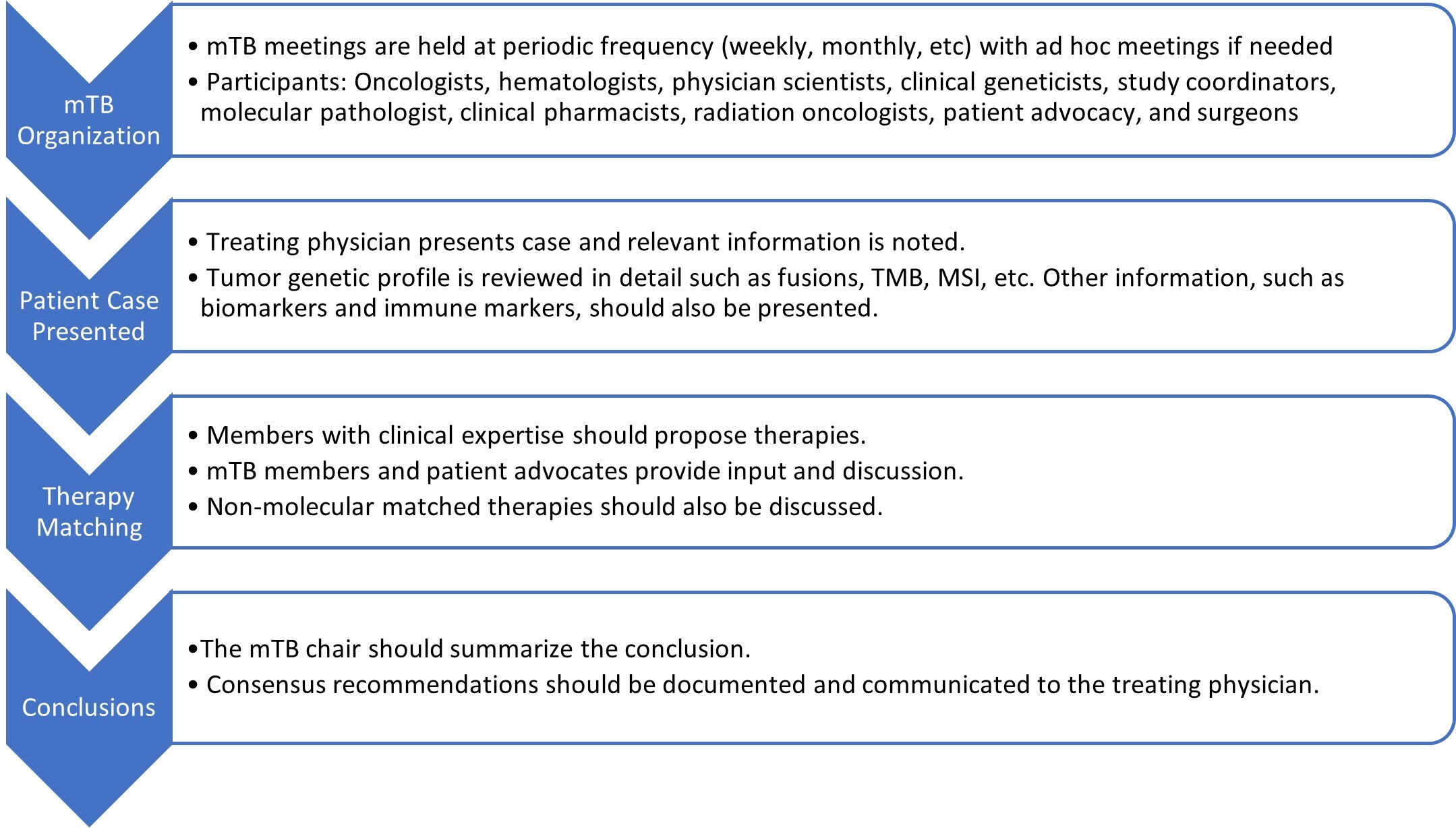
mTBs are crucial to enable PHC, bringing together multidisciplinary experts to analyze complex tumor-specific genetic data. Commonly, mTBs consist of oncologists, pathologists, pharmacists, and molecular scientific experts at their core. A recent systematic literature review looking at 35 mTB publications shows more insights into mTB participation: oncologists (100%), pathologists (91%), geneticists (69%), bioinformatics (38%), clinical molecular biologists (25%), pharmacists (22%), and bioethicists (9%) (Figure 1).3 Classically, mTB participation is in person. However, with the community embracing virtual meetings, studies have shown a 46% increase in stakeholder participation and a 20% increase in overall cases presented. Usual tumor types presented at mTBs include sarcomas (21%), breast cancers (20%), brain tumors (15%), GYN tumors (14%), and unknown/other tumors (31%). This multidisciplinary and collaborative approach ensures the integration of the latest scientific discoveries into diagnosis and clinical care resulting in significant improvement in patient outcomes.4

Figure 1: Molecular tumor board workflow. Ultimately, the treating physician will make the final treatment decision.
Patient Impact of mTBs
A recent systematic literature review looking at 14 reported mTB studies involving 3,328 patient cases and patient outcomes showed healthcare providers adopted mTB-therapy-based recommendations 22% - 43% of the time with clinical benefit ranging from 42% - 100% over nonadoption of mTB recommendations.5 While it’s challenging to understand the reasons for such a wide range in clinical benefit, some examples include the lack of actionable mutations, rapidly progressive disease, and patient inability to participate in clinical trials. In addition, only two of the studies in this literature review were prospective by design. One such study showed improved progression-free survival (PFS) in 44 patients adopting mTB-genomically targeted therapy recommendations, compared to 57 patients not adopting mTB recommendations (mean 86 days vs 49 days, hazard ratio: 0.55, 95% CI 0.37 - 0.84). Interestingly, in the other prospective study noted in this review, 79 patients received a mTB-recommended therapy and did not show a significant clinical benefit difference compared with standard of care. The authors noted reasons including insufficient DNA extraction for NGS and a significant amount of heavily pretreated patients rejecting any additional therapy and/or rapid deterioration. Additional notable positive outcome studies where mTB-based recommendations improved overall survival compared to physician’s treatment of choice include: 1) a group in San Diego (hazard ratio: 0.69, 95% CI 0.49 - 0.98; P=0.036) and a group in Hong Kong (12.7 months compared to 5.2 months, P=0.0073).6-7 While there are multiple studies in the US and globally (eg, NCI-MATCH, PROFILER, PRISM, SAFIR, CATCH, TuPro, WINTHER, TAPUR, IMPACT2) looking at genomically defined biomarkers matched to targeted agents and leveraging mTB recommendations, more studies are needed to specifically understand the impact of mTBs on therapy adoption by patients and related outcomes.4 One such effort from SWOG Cancer Research Network (S2018CD, NCT05455606) is randomizing participation to physician’s treatment of choice compared to recommendations from a mTB.8 The primary objective is to understand if mTBs increase adoption of targeted therapies with multiple secondary objectives such as patient survival.
Selected Challenges of mTB
Variant Calling and Evidence Levels
Even with the clinical utility and patient outcome benefits from mTBs as shown in some studies, there remains significant heterogeneity between mTB-based recommendations. One study assessing the differences of mTB recommendations based on five simulated patient cases sent to five institutions across four countries showed significant heterogeneity between variant interpretation and final patient recommendations.9 To address some of these challenges, multiple groups developed publicly available knowledgebases of variant-actionability (eg, OncoKB, CKB, MTBP) (Table 1).10-12 In addition, joint guidelines from CAP/ASCO/AMP were developed to address the synthesis of evidence potential from multiple databases such as genomic, reference, population, cancer-specific, constitutional-variant, and bespoke-lab in an evidence framework of clinical impact significance.13 This multi-tier framework classifies a variant across pillars including therapeutic, diagnostic, and prognostic. Other evidence frameworks exist including OncoKB, PODS, NCI-MATCH, ESCAT, for example.10, 14-16 Interestingly, one group showed a high discordant rate between molecular-profiling vendor reports compared to corresponding mTB therapeutic recommendations (229 out of 502, 45.6%), with the highest rationale for discordance being low level of evidence.17
Table 1: Summary of publicly available precision oncology knowledgebases. Li X, Warner JL. A review of precision oncology knowledgebases for determining the clinical actionability of genetic variants. Front Cell Dev Biol. 2020;8:48. doi:10.3389/fcell.2020.00048 Licensed under Creative Commons Attribution License (CC BY).
| Knowledgebase | Variant annotation | Drug availability | Trial matching | Literature citation | Website |
| CGI | Yes | Yes | No | Yes | cancergenomeinterpreter.org |
| CIViC | Yes | Yes | No | Yes | civicdb.org |
| HemOnc.org | Yes | Yes | No | Yes | hemonc.org |
| JAX CKB+ | Yes | Yes | Yes* | Yes | ckb.genomenon.com |
| MCG | Yes | Yes | Yes | No | mycancergenome.org |
| OncoKB | Yes | Yes | No | Yes | oncokb.org |
| PCT | Yes | Yes | Yes | Yes | pct.mdanderson.org |
| PMKB | Yes | Yes | No | Yes | pmkb.weill.cornell.edu |
| Drug-associated knowledgebases | |||||
| Genomics of drug sensitivity in cancer | Yes | Yes | No | No | cancerrxgene.org |
| PharmGKB | Yes | Yes | No | No | pharmgkb.org |
| Therapeutic target databases | Yes | Yes | No | Yes | db.idrblab.net/ttd |
*Available only with subscription to CKB BOOST. +Partially publicly available databases.
Turn Around Time
Another key challenge is turn-around time (TAT) from assessment to patient treatment. The tissue acquisition-molecular profiling-mTB-treatment process can be quite long. Typically, the longest part of this process, tumor profiling, typically takes 2 - 3 weeks on top of potentially needing to acquire the tissue block if not readily available.18 In one NSCLC study where the authors measured where they see patient attrition moving through the PHC assessment-treatment paradigm, they noted less than 40% of patients were eligible to receive mTB-recommended therapies due to molecular profiling time and bridging therapy was needed.2 In addition, patients may lose the opportunity for mTB-recommended therapies as their performance status changes and may miss clinical trial opportunities when slots are no longer available. Some institutions have adopted a parallel ctDNA approach to speed up results, but in general, tissue-based TAT profiling times need to be improved.19
Challenges of Integrating Pathology into Multi-disciplinary Care Team
As pathology’s role continues to evolve in the context of PHC and mTBs, additional challenges surface. One study reviewed 659 responses from clinical care team members across a variety of cancer program settings to assess opportunities for pathology integration into multi-disciplinary teams.20 The top five reported challenges include: 1) reimbursement/coverage of testing, 2) insufficient quantity of testing material, 3) turnaround time, 4) test selection and ordering, and 5) communication across the team.
Reimbursement Challenges
Key challenges pertaining to NGS and mTB reimbursement include complex and heterogenous reimbursement policies from insurers, coding and billing issues, and insurers’ perception of value and impactful evidence.21 Medical necessity criterion for molecular oncology tests, especially large panels, must include proven analytical and clinical validity along with clinical utility. Differential patient outcomes based on the test result and subsequent changes in patient management measured against any risks associated with the test are components of clinical utility. Lack of robust evidence in these areas can limit the reimbursement for molecular tests. In addition, pathologists typically spend a significant amount of time preparing for mTBs, which doesn’t involve direct patient care and may be harder to justify reimbursement.
mTB Maturity Assessment
To address some of these challenges, one group recently published a validated mTB assessment tool, which evaluates the maturity of areas (access, consultation, technology, and evidence) in which mTB standardization may help.22 The approach involves a derived mTB maturity model, a 59-question scientific survey, and a five-level mTB maturity scoring algorithm. After deploying the assessment model across 20 institutions globally, the authors noted an average maturity score of 3.3 out of 5 (range 2 - 4.3) with academic institutions showing a significantly higher score than non-academic institutions (3.7 vs 3.1, P = 0.018). Domain areas in which academic institutions scored higher included consultation, evidence, and technology, whereas access to therapies and tests was similar across settings, highlighting potential upstream operational challenges in non-academic settings.
Pathology: What Can We Do?
There are a few considerations for pathology to more effectively support mTBs and the PHC paradigm. Using standardized/guidelines and integrating into laboratory policies/procedures may be useful. This idea includes support of tissue adequacy assessment for molecular testing, ranging from the time of sample acquisition (rapid-onsite evaluation, ROSE) for interventional and/or complicated biopsies to adopting standards of tissue adequacy before samples are sent for molecular testing.23 This also means adopting a communication approach with multi-disciplinary stakeholders when samples are not adequate. In addition, we recommend leveraging guidelines to integrate into lab policies and procedures pertaining to technical deployment of NGS assays, bioinformatical analysis, and evidence frameworks, particularly related to the components of the diagnostic pillar.24 In addition, we recommend considering improving TAT range from straightforward activities such as an agreed communication approach between multi-disciplinary stakeholders, to perhaps considering procedures to order NGS right when the sample is received, to certain reflexive approaches when a patient recurs. The bottom line: it’s better to over-communicate with stakeholders and understand the patient plan than not communicate enough.
mTB reimbursement is another significant challenge that requires a larger effort, including standardization of payer reimbursement policies, coding system improvements, and more evidence of cost-effectiveness. While reimbursement is a larger effort than an individual pathology laboratory, we do recommend at least having the multi-disciplinary care team, typically the oncologist, note within the electronic health record they have ordered a molecular tumor board. Such orders are sometimes helpful in justifying downstream pathology reimbursement.
In summary, mTBs are an evolving and critical component of PHC. While challenges, approaches, and evidence continue to develop, it is critical for pathology to innovate and have a strong voice in their evolution.
References
- Jameson JL, Longo DL. Precision medicine - personalized, problematic, and promising. N Engl J Med. 2015;372:2229-2234. doi:10.1056/NEJMsb1503104
- Tsimberidou AM, Sireci A, Dumanois R, Pritchard D. Strategies to address the clinical practice gaps affecting the implementation of personalized medicine in cancer care. JCO Oncol Pract. 2024;20(6):761-766. doi:10.1200/OP.23.00601.
- Luchini C, Lawlor RT, Milella M, Scarpa A. Molecular tumor boards in clinical practice. Trends Cancer. 2020;6(9):738-744. doi:10.1016/j.trecan.2020.05.008.
- Tsimberidou A, et al. Molecular tumour boards - current and future considerations for precision oncology. Nat Rev Clin Oncol. 2023;20(12):843-863. doi:10.1038/s41571-023-00824-4.
- Larson KL, et al. Clinical outcomes of molecular tumor boards: A systematic review. JCO Precis Oncol. 2021;5:PO.20.00495. doi:10.1200/PO.20.00495.
- Kato S, et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun. 2020;11(1):4965. doi:10.1038/s41467-020-18613-3. PMID: 33009371; PMCID: PMC7532150.
- Helali A, et al. The impact of the multi-disciplinary molecular tumour board and integrative next generation sequencing on clinical outcomes in advanced solid tumours. Lancet Reg Health West Pac. 2023:36:100775. doi:10.1016/j.lanwpc.2023.100775.
- Southwest Oncology Group. Trial s2108cd. https://www.swog.org/clinical-trials/s2108cd
- Rieke D. et al. Comparison of treatment recommendations by molecular tumor boards worldwide. JCO Precis Oncol. 2018;2:1-14. doi:10.1200/PO.18.00098.
- Memorial Sloan Kettering Cancer Center. OncoKB. https://www.oncokb.org/
- The Jackson Laboratory. Clinical Knowledge Database. https://www.jax.org/clinical-genomics/ckb
- Tamborero D, et al. Support systems to guide clinical decision-making in precision oncology: The Cancer Core Europe Molecular Tumor Board Portal. Nat Med. 2020;26(7):992-994. doi:10.1038/s41591-020-0969-2.
- Li MM, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19(1):4-23. doi:10.1016/j.jmoldx.2016.10.002.
- Johnson A, et al. The right drugs at the right time for the right patient: the MD Anderson precision oncology decision support platform. Drug Discov Today. 2015;20(12):1433-8. doi:10.1016/j.drudis.2015.05.013.
- O'Dwyer PJ, et al. The NCI-MATCH trial: lessons for precision oncology. Nat Med. 2023;29(6):1349-1357. doi:10.1038/s41591-023-02379-4.
- Mateo J, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29(9):1895-1902. doi:10.1093/annonc/mdy263.
- Walters M, et al. Quantifying the value of the molecular tumor board: discordance recommendation rate and drug cost avoidance. JCO Precis Oncol. 2022;6:e2200132. doi:10.1200/PO.22.00132.
- Basse C, et al. Relevance of a molecular tumour board (MTB) for patients' enrolment in clinical trials: experience of the Institut Curie. ESMO Open. 2018;3(3):e000339. doi:10.1136/esmoopen-2018-000339.
- Sánchez NS, et al. Identification of actionable genomic alterations using circulating cell-free DNA. JCO Precis Oncol. 2019;3:PO.19.00017. doi:10.1200/PO.19.00017.
- Plotkin E, et al., Integration of pathology within the multidisciplinary cancer care team. J Clin Ocol. 37, 49-49(2019). doi:10.1200/JCO.2019.37.27_suppl.49
- Deverka P, et al. Clinical integration of next generation sequencing: coverage and reimbursement challenges. J Law Med Ethics. 2014;42:22-41. doi:10.1111/jlme.12160.
- Love T, et al. Development and validation of ACTE-MTB: A tool to systematically assess the maturity of molecular tumor boards. PLoS One. 2022;17(5):e0268477. doi:10.1371/journal.pone.0268477.
- Masood S, Siddiqi A. Cytopathology in focus: ROSE and telecytopathology: a point-of-care test. CAPOnlineToday. 2022.
- Li X, Warner JL. A review of precision oncology knowledgebases for determining the clinical actionability of genetic variants. Front Cell Dev Biol. 2020;8:48. doi:10.3389/fcell.2020.00048

Michael Barnes, MD, FCAP, is a board-certified pathologist director at AbbVie, focused on clinical trial and companion diagnostic development. He completed his medical studies at Ohio State University and AP Residency, Surgical Pathology, Neuropathology, and Post-Doctoral Research Fellowships at University of California, San Francisco. Subsequently, he spent 10 years at Roche in their Ventana and Genentech organizations and more recently at PathAI. He is a member of CAP's PHC Committee. His research interests are integrating digital pathology into clinical trials and efficient biomarker-driven trial approaches.

Erin Cobain, MD, is an Associate Professor of Internal Medicine in the Division of Hematology/Oncology at the University of Michigan. She serves as co-chair of the Breast Cancer Clinical Research Team at the University of Michigan Rogel Cancer Center. Her research focuses on identifying those at high risk of developing breast cancer and personalizing therapy for those with a breast cancer diagnosis through genetic and genomic testing. Dr. Cobain has a particular interest in identifying which patients with breast cancer may benefit most from immunotherapy treatment. She works closely with colleagues at the University of Michigan, as well as the SWOG Cancer Research Network, to develop clinical trials that incorporate tumor biomarker testing to personalize cancer therapy.