- Home
- Member Resources
- Articles
- Molecular Oncology Testing in the Pediatric Population
As in adult oncology, pediatric molecular oncology testing provides diagnostic, prognostic, and therapeutic information derived from tumor genomes. Fundamentally, proper treatment for a patient depends upon an accurate and complete diagnosis of their tumor and is further directed by prognostic and, more recently, therapeutic molecular signatures. While there are some similarities to adult oncology, the biology of pediatric tumors is different, thus pediatric molecular oncology testing must reflect these differences.
Differing Tumor Types and Incidence

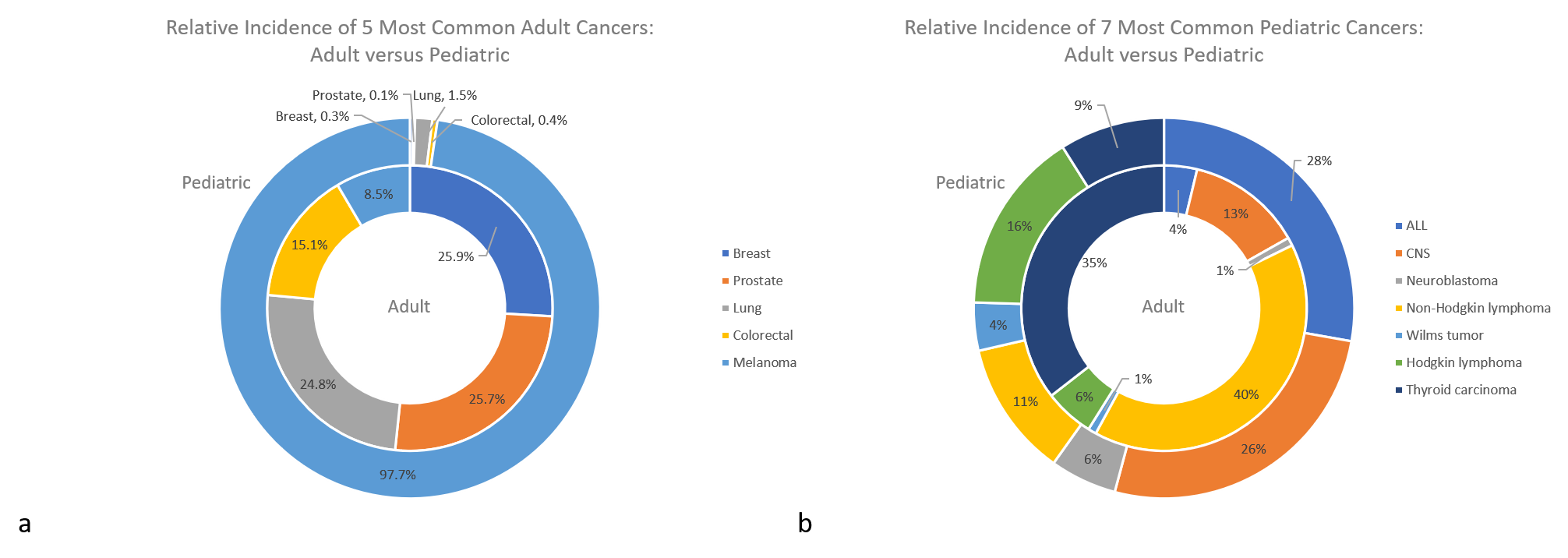
Figure 1: The relative incidence of pediatric and adult tumors when considering the five most common adult cancers (a) and the seven most common pediatric cancers (b). 1,3,4,5,6,20
First, pediatric malignancy is rare, accounting for approximately 1% of all incidences of human cancer, which has complicated research of molecular oncogenesis and its application to pediatric tumors. Direct comparisons are also complicated by the fact that adult cancers are classified by the anatomical site of the primary tumor, while cancers in children and younger adolescents are classified by histology into the 12 major groups of the International Classification of Childhood Cancers system. Absolute numbers notwithstanding, there are significant differences between the proportion and types of tumors that arise between pediatric and adult patients. A review of Surveillance, Epidemiology, and End Results Program (SEER) data shows adults are most commonly affected by carcinomas while pediatric patients develop predominantly hematologic and non-epithelial solid tumors (Figure 1).1,2
Differing Molecular Drivers

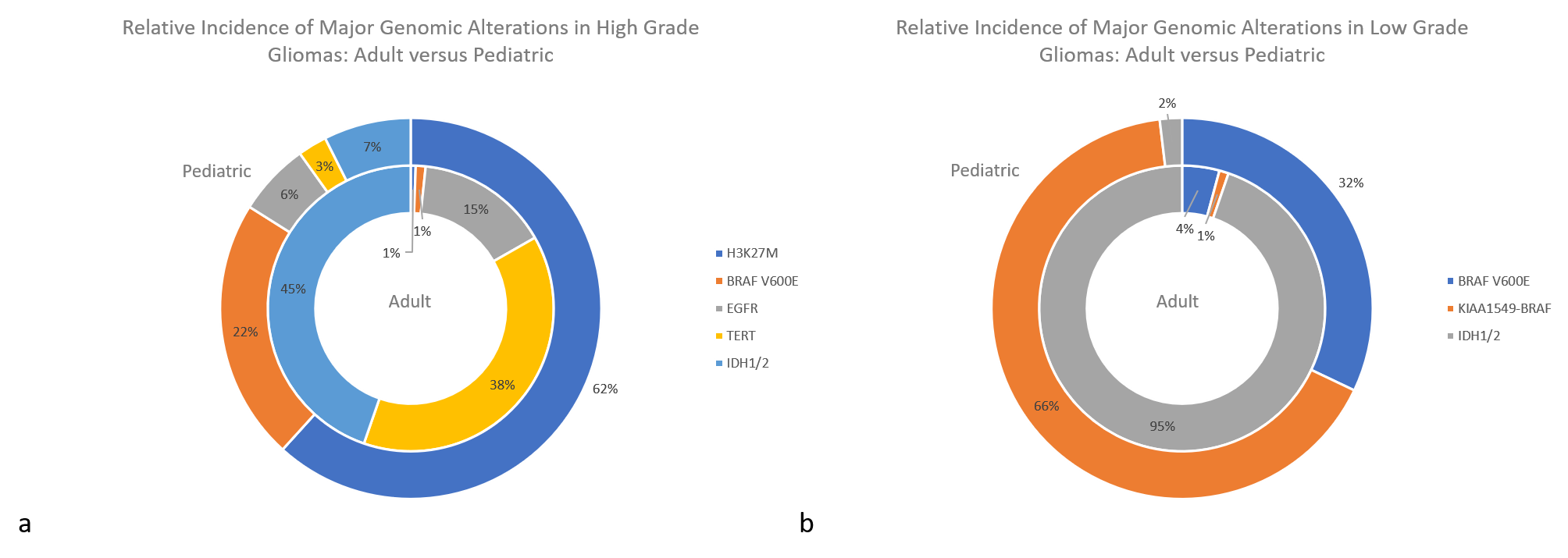
Figure 2: The relative incidence of major genomic alterations in high grade gliomas (a) and low-grade gliomas (b). 7,8,9,10,11,12,13,14,15,16,17,18,21,22
With these differences in tumor type come different molecular features promoting tumorigenesis. For example, different molecular alterations drive gliomagenesis between adult and pediatric populations, with some occurring almost exclusively in one group versus the other (Figure 2). Adult high-grade gliomas are glioblastomas (WHO grade IV), while pediatric high-grade gliomas include WHO grade III and IV tumors (including diffuse midline gliomas, H3 K27M-mutant). Adult low-grade gliomas refer to WHO grade II and III, and pediatric low-grade refers to WHO grade I and II.
IDH1/2 variants are identified in most adult gliomas and are only rarely seen in pediatric patients. The vast majority of pediatric IDH mutations are in older adolescents, representing the early tail of adult cases, and those identified under age 14 are exceedingly rare. Conversely, H3 K27M-mutant midline gliomas represent the bulk of pediatric high-grade tumors and are only very rarely seen in adult patients. When found in adults, they have a less aggressive clinical course, suggesting differences in the underlying biology that remains to be more clearly understood.7
In addition to the H3 K27M-mutant midline gliomas, the World Health Organization classifications are increasingly recognizing exclusively or mostly pediatric entities with distinct molecular features, including several hematopoietic entities. Pediatric-type follicular lymphoma (PTFL) lacks the characteristic t(14;18) of most follicular lymphomas. Similarly, mutations in KMT2D, CREBBP, and EZH2 are also not seen with PTFL, but instead show a predominance of MAP2K1 mutations. Conversely, genetic aberrations of 1p36 are common to both types. Large B-cell lymphoma with IRF4 rearrangement is defined by rearrangement of IRF4, mostly with IGH, and is cytogenetically cryptic. Burkitt-like lymphoma with 11q aberration resembles Burkitt lymphoma in multiple respects but lacks MYC rearrangements, instead showing an 11q aberration characterized by proximal interstitial gains at 11q23 and telomeric losses. Pediatric nodal marginal zone lymphoma shows similar genetic features to its adult counterpart, including occasional trisomies of chromosomes 18 or 3 and clonal rearrangements of the IGH region, but future differences may become apparent in the various regions of the IGH cluster that are involved.19
While some tumor types – such as Hodgkin lymphoma and T-lymphoblastic lymphoma – appear to show very little genomic difference between pediatric and adult populations, many others do28 30. A sampling is summarized in the table below:
| Tumor Type | Adult | Pediatric |
|---|---|---|
| Breast cancer | >90% carcinoma with ER, PR, and/or HER2 expression | ~40% mesenchymal, predominantly Phyllodes tumors |
| Colorectal adenocarcinoma | ~15% mucinous | ~50% mucinous with higher rates of MSI and MMR mutations |
| Prostate cancer | >95% carcinoma | >99% rhabdomyosarcoma |
| Differentiated thyroid carcinoma | Predominantly BRAF and RAS mutations | Higher frequency of RET and NTRK1/2/3 fusions |
| Diffuse large B-cell lymphoma | mix of GCB and ABC subtypes; occasional BCL2/6 and/or MYC rearrangements | mostly GCB subtype; rarely rearranged BCL2/6, higher frequency of MYC abnormalities |
| B-lymphoblastic leukemia | BCR-ABL1 fusion more frequent | ETV6-RUNX1 and hyperdiploidy more common; iAMP21 almost exclusively pediatric |
| Melanoma | predominantly BRAF, RAS and NF1 mutations | NRAS p.Q61 (giant congenital melanoma), multiple gene fusions (spitzoid melanomas) |
| MSI = microsatellite instability, MMR = mismatch repair, GCB = germinal center B-cell, ABC = activated B-cell References: 4,6,19,23,24,25,26,27,28,29,31,32,33,34,35,37 | ||
With their own various chromosomal alterations, pediatric embryonal tumors are rare to absent in adults. Wilms tumors are frequently risk stratified using chromosomal segmental alterations such as 1q gain and 1p and 16q loss of heterozygosity (LOH). Neuroblastomas are similarly queried for 1p/11q LOH and MYCN amplification. Retinoblastomas show deletions involving RB1 as well as MYCN amplifications. Medulloblastomas may show monosomy 6 in WNT-activated subtypes, 10q LOH in SHH-activated subtypes, and amplifications of MYC or MYCN in multiple subtypes, among various other genomic aberrations. Several other rarer pediatric embryonal tumors are similarly investigated, often by combinations of g-banding karyotypes, fluorescence in situ hybridization (FISH), chromosomal microarray, and immunohistochemistry. Next-generation sequencing (NGS) platforms are becoming increasingly useful for these tumors although reliable copy number variant detection, particularly with large chromosomal segmental alterations remains a challenge on most NGS assays.21,22,34
Additionally, many pediatric sarcomas, including the “small round blue cell tumors”, are characterized by gene rearrangements. Rhabdomyosarcomas are necessarily routinely tested for FOXO1 rearrangement, usually by FISH. Ewing sarcomas have historically been confirmed by immunohistochemistry and detection of EWSR1 rearrangement by FISH; however, the discovery of several other tumor types with EWSR1 rearrangements that may confound the differential diagnosis have made detection of the fusion partners more important. The newer group of “Ewing-like” or undifferentiated round cell sarcomas are defined by their relevant gene fusions such as CIC-rearranged sarcomas, most commonly with DUX4, and BCOR-rearranged sarcoma, usually with CCNB3. NGS assays with the ability to query RNA and investigate multiple potential fusion partners simultaneously have found particular utility for diagnosis of these tumors.38
Differing Cancer Genes

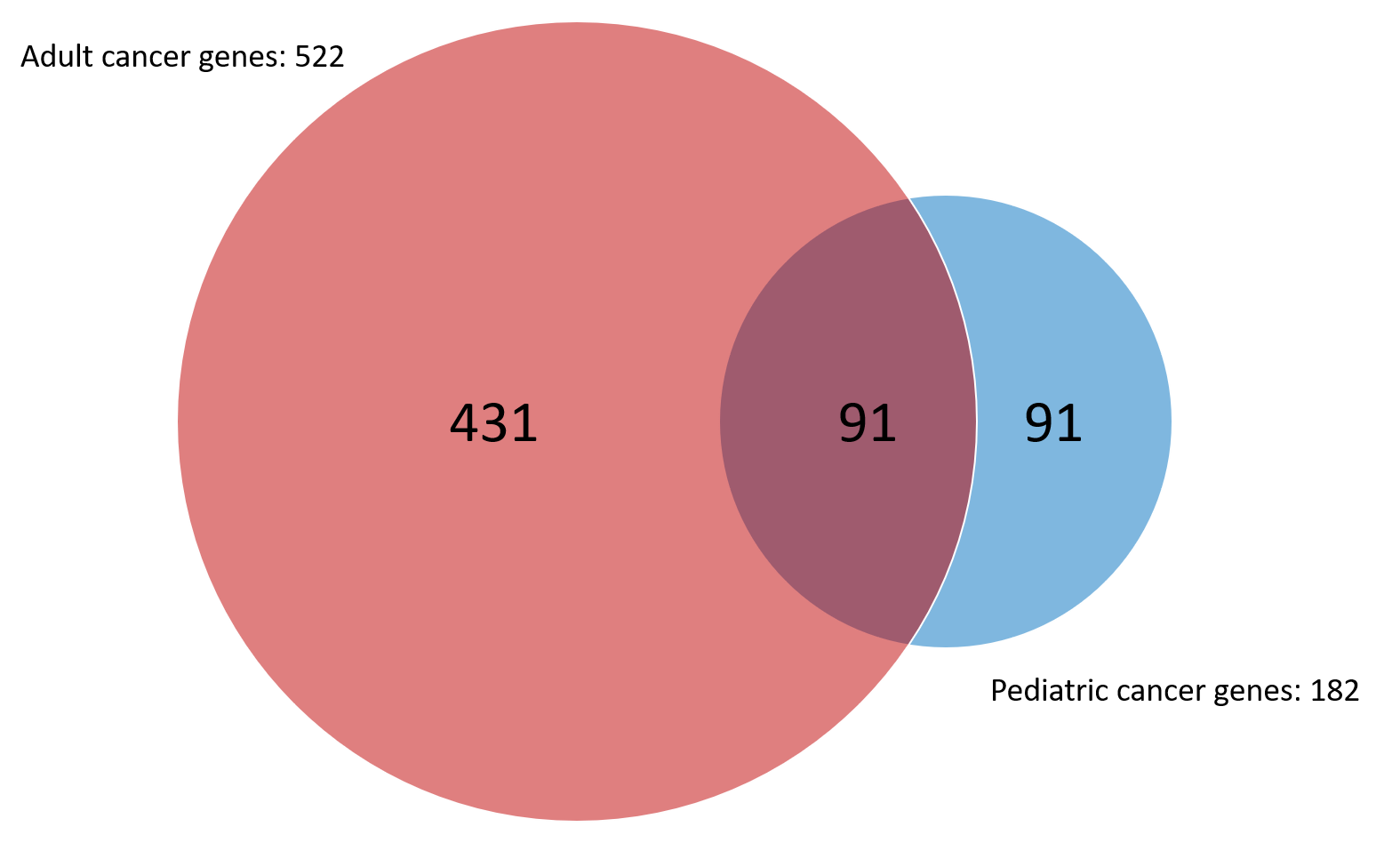
The increasing availability and diminishing costs of large sequencing projects have led to many publications investigating the genomic landscapes of adult and pediatric tumors alike. Recent pan-cancer assessments have shown half of proven and suspected pediatric cancer-related genes do not overlap with adult genes (Figure 3).
Testing Methods
The classic mainstays of pediatric malignancy testing include FISH and chromosomal analysis, particularly in leukemias and sarcomas. FISH remains a well-established technique for the detection of translocations, either via detection of normal structure being lost (breakapart probes) or abnormal structures being formed (fusion probes). Reverse transcription PCR followed by sequencing is also frequently employed, although the agnostic nature of FISH breakapart probes towards the fusion partner gene of particularly promiscuous oncogenes (e.g., KMT2A) carries an advantage. Karyotyping, despite its relative technical complexity and slow turnaround times, remains a mainstay and indeed is required for ploidy assessment and resolution of atypical FISH results. Chromosomal microarray testing, well-established in leukemia testing, has also become more established in solid tumors for copy number assessment as described earlier with more recent advances that have improved the interpretation of tumor tissue retrieved from formalin-fixed paraffin embedded (FFPE) blocks. Laboratories are increasingly using more NGS assays although some technical limitations, such as copy number variant and large chromosomal structural alteration detection, and accessibility and cost have been factors limiting widespread adoption.
Laboratory Considerations
Molecular pathology laboratories will benefit from considering their respective testing populations. If pediatric malignancies are routinely tested, access to the various modalities discussed above will be essential either as an in-house capability or via partnership with a reference laboratory. NGS platforms will continue to become more practical and laboratories will want to pay special attention to developing broad gene rearrangement detection and querying of relevant pediatric cancer genes.
Molecular testing increasingly offers more reliable and defined diagnoses as well as prognostic implications and therapeutic choices - all factors that will further refine the information available to clinicians, patients, and their families to make the right choice for their particular situation. Recognizing and addressing the different needs of pediatric patients will allow molecular pathology laboratories to be a key partner in that process.
References:
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014.
- Vogelstein B, et al. Cancer genome landscapes. Science. 2013 Mar 29;339(6127):1546-58. PMID: 23539594
- PDQ® Adult Treatment Editorial Board. PDQ Breast Cancer Treatment (Adult). Bethesda, MD: National Cancer Institute. Updated 09/02/2020. Available at: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq. Accessed 11/30/2020. PMID: 26389406
- Rojas Y, et al. Primary malignant pulmonary tumors in children: a review of the national cancer data base. J Pediatr Surg. 2015 Jun;50(6):1004-8. PMID: 25812444
- PDQ® Adult Treatment Editorial Board. PDQ Colon Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated 01/25/2021. Available at: https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq. Accessed 02/03/2021. PMID: 26389297
- PDQ® Adult Treatment Editorial Board. PDQ Melanoma Treatment. Bethesda, MD: National Cancer Institute. Updated 02/05/2021. Available at: https://www.cancer.gov/types/skin/hp/melanoma-treatment-pdq. Accessed 02/06/2021. PMID: 26389469
- Louis, DN, et al. WHO Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer, 2016.
- Ryall S, et al. Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell. 2020 Apr 13;37(4):569-583.e5. PMID: 32289278
- Mackay A, et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell. 2017 Oct 9;32(4):520-537.e5. PMID: 28966033
- PDQ® Adult Treatment Editorial Board. PDQ Adult Central Nervous System Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated 01/13/2021. Available at: https://www.cancer.gov/types/brain/hp/adult-brain-treatment-pdq. Accessed 02/01/2021. PMID: 26389419
- Hao Z, Guo D. EGFR mutation: novel prognostic factor associated with immune infiltration in lower-grade glioma; an exploratory study. BMC Cancer. 2019 Dec 4;19(1):1184. PMID: 31801484
- Behling F, Schittenhelm J. Oncogenic BRAF Alterations and Their Role in Brain Tumors. Cancers (Basel). 2019 Jun 8;11(6):794. PMID: 31181803
- Zheng S, et al. Prospective Clinical Sequencing of Adult Glioma. Mol Cancer Ther. 2019 May;18(5):991-1000. PMID: 30926639
- Frazão L, et al. BRAF V600E mutation and 9p21: CDKN2A/B and MTAP co-deletions - Markers in the clinical stratification of pediatric gliomas. BMC Cancer. 2018 Dec 17;18(1):1259. PMID: 30558563
- Bell EH, et al. Association of MGMT Promoter Methylation Status With Survival Outcomes in Patients With High-Risk Glioma Treated With Radiotherapy and Temozolomide: An Analysis From the NRG Oncology/RTOG 0424 Trial. JAMA Oncol. 2018 Oct 1;4(10):1405-1409. PMID: 29955793
- Rodriguez FJ, et al. Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients. Am J Surg Pathol. 2014 Aug;38(8):1058-70. PMID: 24805856
- Paugh BS, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010 Jun 20;28(18):3061-8. PMID: 20479398
- LaStarza R, et al. “TERT Mutations in Glioma: ESMO Biomarker Factsheet.” OncologyPRO | Educational Portal for Oncologists, OncologyPRO, 25 Jan. 2019, https://oncologypro.esmo.org/education-library/factsheets-on-biomarkers/tert-mutations-in-glioma. Accessed 02/15/2021.
- Swerdlow SH, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer, 2017.
- PDQ® Adult Treatment Editorial Board. PDQ Non-Small Cell Lung Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated 11/19/2020. Available at: https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq. Accessed 11/30/2020. PMID: 26389304
- PDQ® Pediatric Treatment Editorial Board. PDQ Neuroblastoma Treatment. Bethesda, MD: National Cancer Institute. Updated 06/08/2020. Available at: https://www.cancer.gov/types/neuroblastoma/hp/neuroblastoma-treatment-pdq. Accessed 11/30/2020. PMID: 26389190
- PDQ® Pediatric Treatment Editorial Board. PDQ Wilms Tumor and Other Childhood Kidney Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated 11/05/2020. Available at: https://www.cancer.gov/types/kidney/hp/wilms-treatment-pdq. Accessed 11/30/2020. PMID: 26389282
- Holm M, et al. Primary breast sarcoma: A retrospective study over 35 years from a single institution. Acta Oncol. 2016 May;55(5):584-90. PMID: 26586158
- Ashlock R, Johnstone JAS. Treatment modalities of bladder/prostate rhabdomyosarcoma: a review. Prostate Cancer Prostatic Dis. 2003;6(2):112-20. PMID: 12806368
- Neville HL, et al. Incidence and outcomes of malignant pediatric lung neoplasms. J Surg Res. 2009 Oct;156(2):224-30. PMID: 19631347
- Zhang X, et al. B lymphoblastic leukemia/lymphoma: new insights into genetics, molecular aberrations, subclassification and targeted therapy. Oncotarget. 2017 Jul 15;8(39):66728-66741. PMID: 29029550
- Marks DI, Rowntree C. Management of adults with T-cell lymphoblastic leukemia. Blood. 2017 Mar 2;129(9):1134-1142. PMID: 28115371
- Girardi T, et al. The genetics and molecular biology of T-ALL. Blood. 2017 Mar 2;129(9):1113-1123. PMID: 28115373
- Bush NAO, Chang S. Treatment Strategies for Low-Grade Glioma in Adults. J Oncol Pract. 2016 Dec;12(12):1235-1241. PMID: 27943684
- PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Hodgkin Lymphoma Treatment. Bethesda, MD: National Cancer Institute. Updated 10/05/2020. Available at: https://www.cancer.gov/types/lymphoma/hp/child-hodgkin-treatment-pdq. Accessed 12/05/2020. PMID:26389170
- PDQ® Adult Treatment Editorial Board. PDQ Prostate Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated 12/10/2020. Available at: https://www.cancer.gov/types/prostate/hp/prostate-treatment-pdq. Accessed 12/15/2020. PMID: 26389471
- PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Colorectal Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated 10/06/2020. Available at: https://www.cancer.gov/types/colorectal/hp/child-colorectal-treatment-pdq. Accessed 10/30/2020.
- PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Melanoma Treatment. Bethesda, MD: National Cancer Institute. Updated 10/06/2020. Available at: https://www.cancer.gov/types/skin/hp/child-melanoma-treatment-pdq. Accessed 10/30/2020.
- Jones DTW, et al. Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Nat Rev Cancer. 2019 Aug;19(8):420-438. PMID: 31300807
- PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Thyroid Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated 10/06/2020. Available at: https://www.cancer.gov/types/thyroid/hp/child-thyroid-treatment-pdq. Accessed 10/29/2020. PMID: 26389315
- Repana D, et al. The Network of Cancer Genes (NCG): a comprehensive catalogue of known and candidate cancer genes from cancer sequencing screens. Genome Biol. 2019 Jan 3;20(1):1. PMID: 30606230
- PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Acute Lymphoblastic Leukemia Treatment. Bethesda,MD: National Cancer Institute. Updated 11/25/2020. Available at: https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq. Accessed 12/02/2020. PMID:26389206
- Antonescu CR, et al. WHO Classification of Tumours of Soft tissue and bone. International Agency for Research on Cancer, 2020.

Damon R. Olson, MD, FCAP, is Molecular Pathology Program Director and Associate Medical Director of Anatomic Pathology at Children's Minnesota. He is board-certified in Molecular Genetic Pathology, Pediatric Pathology, and Anatomic and Clinical Pathology. He serves on the Personalized Health Care Committee of the College of American Pathologists and the Education Committee of the Society of Pediatric Pathology.