- Home
- Member Resources
- Articles
- How Good are COVID-19 (SARS-CoV-2) Diagnostic PCR Tests?
After reading the article, hear more background from the author on the CAPcast, How Good are COVID-19 (SARS-CoV-2) Diagnostic PCR Tests?
I get asked this question frequently by both medical and non-medical people. People hear numbers thrown around that range anywhere from 70% to 99% and don’t know what to believe. Unfortunately, the answer is not simple and requires understanding both the impact of pre-analytic and analytic factors AND the difference between analytic and clinical performance of a test.
Analytic Versus Clinical Performance
The analytic performance of PCR based tests is good, with most assays able to detect 500-5000 copies of viral RNA/mL1 near 100% of the time (analytical sensitivity) and most tests do not cross react with other viruses, so the analytical specificity is near 100% also. However, clinical performance (how well the test predicts disease) is lower. Clinical performance is affected not only by the analytic performance, but also by biologic and pre-analytic factors.
Clinical performance of a test is typically determined compared to a gold standard (the accepted best way of diagnosing a condition); however, SARS-CoV-2 is new and we don’t have a true gold standard. Studies have used “gold standards” ranging from CT evidence of pneumonia in a pandemic area (not perfect as there are other causes of pneumonia, even in a pandemic) to a combination of clinical features, imaging, and positive follow-up testing (probably the best we are going to get). In these studies, clinical performance ranges and approaches 80% sensitivity and 98-99% specificity when using a good comparator5, so why is the clinical sensitivity so different from the analytical sensitivity?
Biology and Pre-Analytic Factors
The pre-analytic phase of testing refers to everything that happens to a sample before the test is run: sample collection, transportation, and storage. For COVID-19 testing, biology including where the virus is present, how much virus is present, and time from symptom onset also affects how the test performs.1 Sample collection and biology are both critically important, the amount of virus present at an anatomic site may differ so the site of sample collection may affect the test result4 and the quality of the collection will affect how much virus (if present) goes into the sample tube. For example, we and others have seen patients with COVID-19 pneumonia who had a negative to low positive test on a nasopharyngeal sample but a highly positive test on tracheal fluid.4 Biologic variation on where virus is present in the body, may necessitate different sample collection. Other pre-analytic factors, like adverse storage and transportation conditions degrade viral RNA and affect testing but can be controlled with training and good operations.
Bringing it All Together
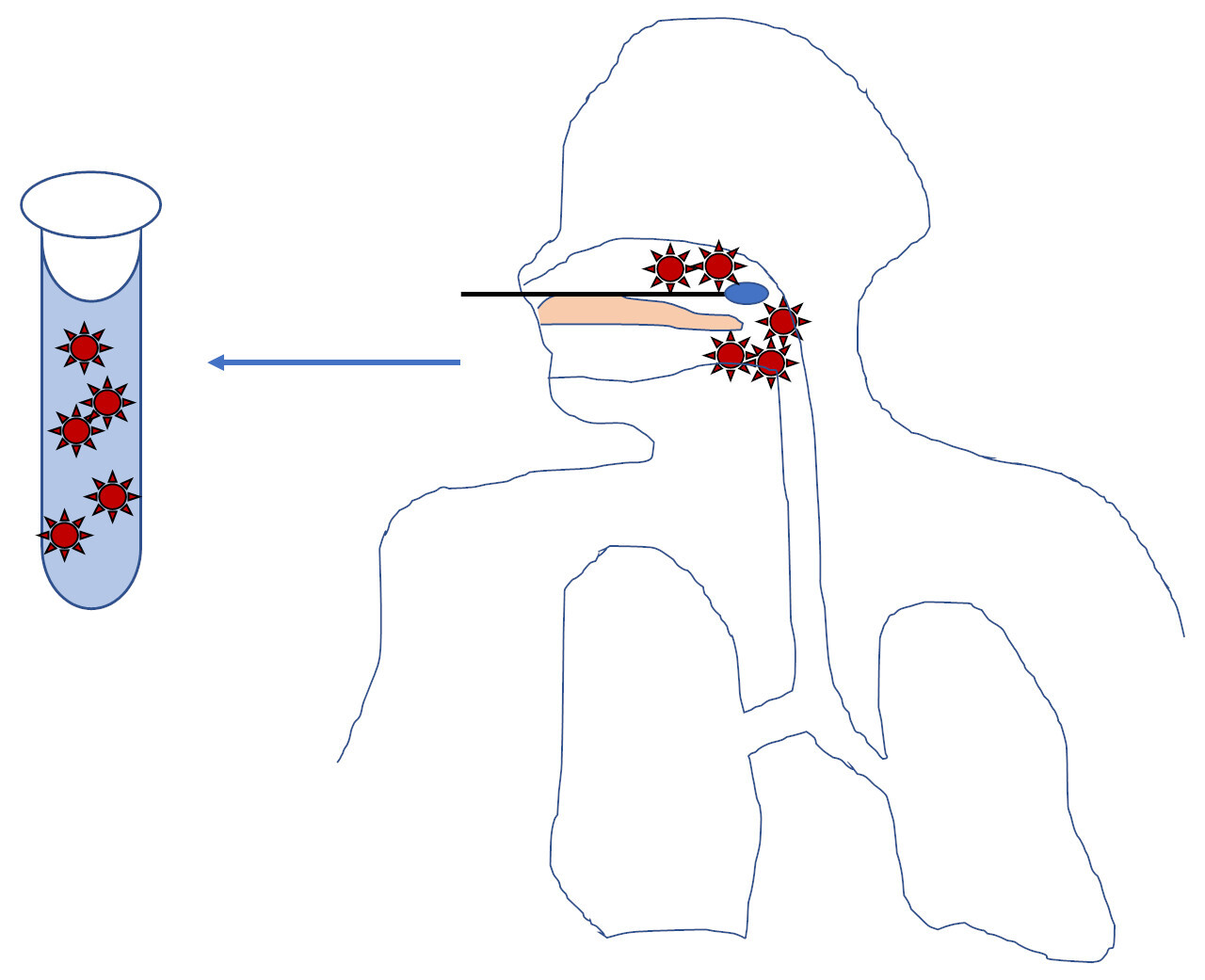
Whether a SARS-CoV-2 test detects clinical disease depends on biologic factors, pre-analytic factors, and analytic performance. Someone with a large amount of virus in their nose/throat will have a positive test with a nose/throat swab (Figure 1).
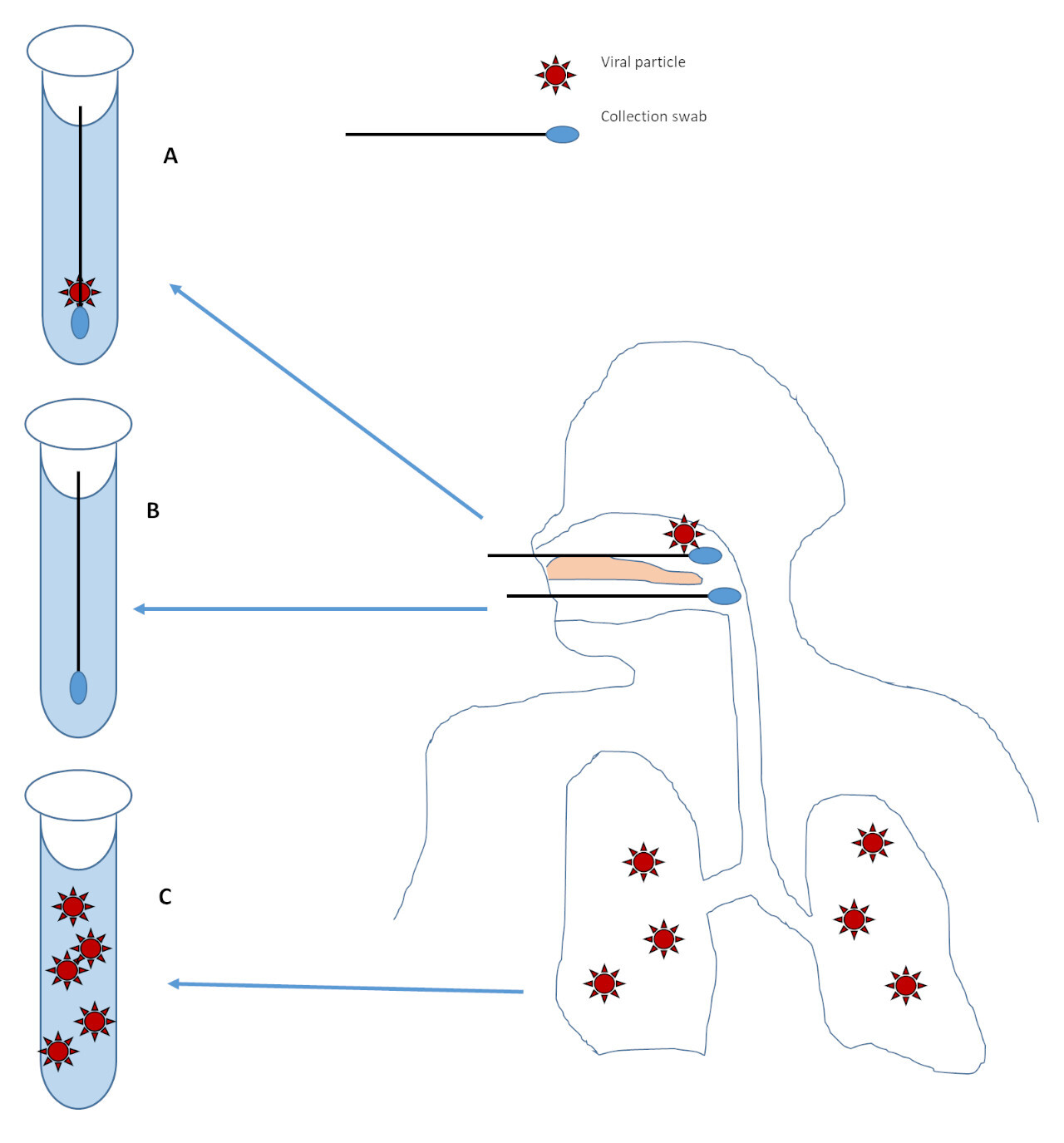
However, someone with little to no virus in their nose or throat may have a negative test even if they have virus somewhere else (like the lungs)3 (Figure 2C). If there is only a small amount of virus present, as may occur later in the disease course2,3, then analytic sensitivity or minor pre-analytic issues may affect viral detection (Figure 2A). If no virus is present at the site of collection, the collection fails to get virus in the sample, or the sample is severely degraded from storage or transport (for example baking in the sun on a car dash) then the test will be negative no matter how sensitive the test is (Figure 2B).
Summary
Analytic performance of many SARS-CoV-2 diagnostic PCR tests approaches 100% at 500-5000 copies/mL; however, clinical performance of testing depends on biology and pre-analytic factors and only approaches 80% sensitivity and 98-99% specificity. Learning more about the biology of SARS-CoV-2 and optimizing pre-analytic factors should improve the clinical performance of SARS-CoV-2 diagnostic testing. If COVID-19 is suspected and testing is negative, re-testing of a clinically affected site may be indicated.
Listen to the podcast
Sophia Yohe, MD, FCAP, director of the University of Minnesota Medical School’s Molecular Diagnostics Laboratory, addressed many questions about diagnostic PCR testing for SARS-CoV-2, the virus that causes COVID-19
References
1. Prinzi, Andrea. False Negatives and Reinfections: The Challenges of SARS-CoV-2 RT-PCR Testing.
https://asm.org/Articles/2020/...
2. Tao Ai, Zhenlu Yang, Hongyan Hou, Chenao Zhan, Chong Chen, Wenzhi Lv, Qian Tao, Ziyong Sun, Liming Xia. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. Feb 26, 2020. https://pubs.rsna.org/doi/pdf/...
3. Wölfel, R., Corman, V.M., Guggemos, W. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469 (2020). https://doi.org/10.1038/s41586...
4. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843–1844. doi:10.1001/jama.2020.3786
5. He JL, Luo L, Luo ZD, et al. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168:105980. doi:10.1016/j.rmed.2020.105980

Sophia Yohe, MD, FCAP is an associate professor at the University of Minnesota and medical director of the molecular diagnostics laboratory and of the University of Minnesota's COVID testing lab. Prior to the COVID pandemic, her research interests included improvement of knowledge in the diagnostic, prognostic, and therapeutic characterization of patients with cancer, in particular hematologic neoplasms and she hopes to return to these pursuits within the next six months.