- Home
- Member Resources
- Articles
- HER2/ERBB2 Testing in Colorectal Cancer
Colorectal cancer (CRC) comprises 10% of all newly diagnosed cancers worldwide and is the second most common cause of death due to cancer in the United States.1,2 The last several decades have seen significant improvement in CRC patient outcomes—reported median overall survival for patients with metastatic colorectal cancer (mCRC) is approximately 30 months, compared to reports of 12 to 15 months in the early 2000s.3,4 These improvements in patient outcomes are derived from a variety of advances in pathology diagnostics, surgical techniques, molecular pathology, and clinical oncology.3 Newer treatment regimens linked to molecular biomarkers such as deficiency in mismatch repair/microsatellite instability, BRAF mutations, neurotrophic tyrosine receptor kinase (NTRK) fusions, and tumor mutation burden have improved outcomes in large numbers of patients.5 Nonetheless, the majority of patients with Stage IV or mCRC still die from their disease. Encouragingly, a growing body of literature has identified that HER2/ERBB2 is a targetable pathway in some mCRC patients compared to other forms of therapy.5-7
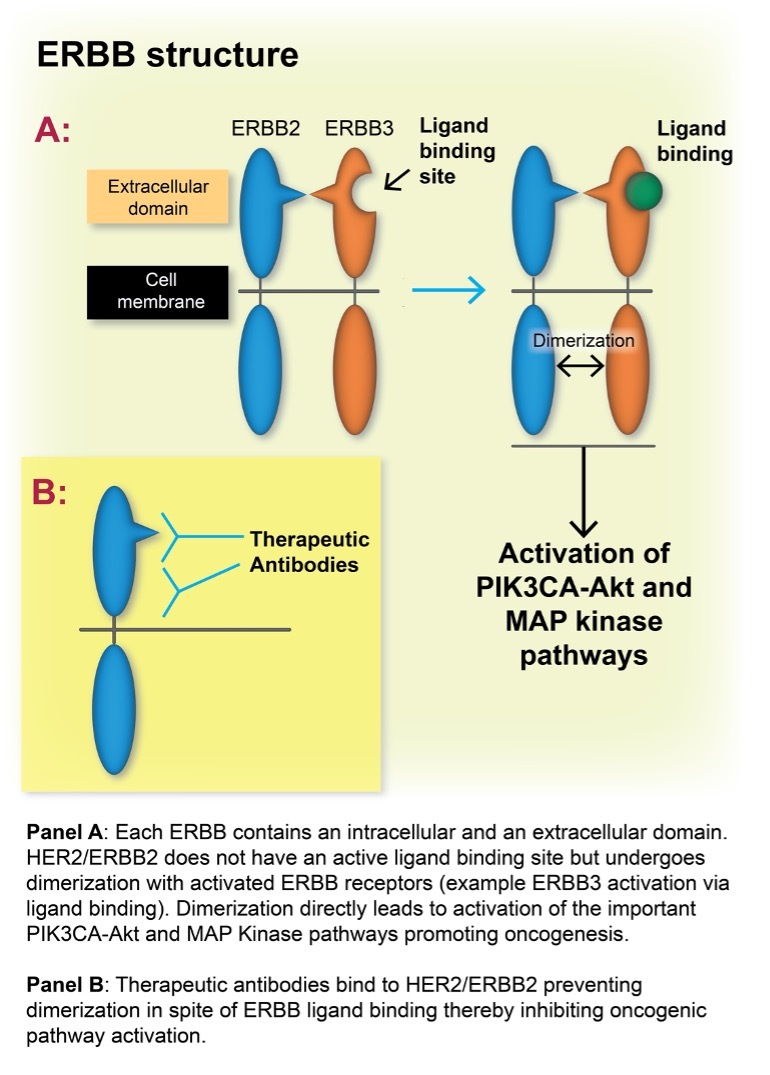
HER2/ERBB2 Cancer Biology and Therapeutics: ERBB receptor tyrosine kinases (RTKs) comprise four members - EGFR/ERBB1, ERBB2, ERBB3, and ERBB4. These RTKs modulate multiple intracellular pathways crucial to cellular metabolism. Two of the main pathways activated by the receptors are the mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K)–AKT pathways. Interestingly HER2/ERBB2 activation does not occur via ligand binding to its receptor but by activation of the other ERBB receptors, leading to dimerization with HER2/ERBB2 (Figure 1). Protein overexpression of ERBB2 in tumors [HER2+], the majority associated with ERBB2 gene amplification, leads to constitutive activation of ERBB2, presumably because of a marked increased receptor concentrations at the plasma membrane – leading to dysregulated cell proliferation.8
The relationship between HER2/ERBB2 mutations and overexpression is complex and a source of ongoing study across a number of cancer systems. For example, a recent analysis stratified HER2/ERBB2 mutations in gastrointestinal cancers based on their associations with gene amplification linked with other cancer molecular features such as microsatellite instability status and tumor mutation burden.9
A number of preclinical and clinical studies have linked HER2/ERBB2 overexpression with poorer responses to anti-EGFR therapies in KRAS/NRAS/BRAF wild type CRCs.7,10 This directly relates to the lack of cellular addiction to EGFR activation of downstream MAPK and PIK3CA pathways due to aberrant signaling via overexpression of HER2/ERBB2.10
Overexpression of HER2/ERBB2 defines cancers susceptible to growth inhibition via anti-HER2 therapy—most well-understood in the treatment of breast cancer, but occurring in varying degrees in multiple cancer types. In the gastrointestinal tract, this was first highlighted as an effective therapeutic option in the landmark trastuzumab for gastric cancer (ToGA) trial of anti-HER2 therapy in HER2+ upper gastrointestinal adenocarcinomas.11
Recently, multiple preclinical studies have investigated the efficacy of anti-HER2 therapies in vitro, identifying that combination therapies of anti-HER2 drugs have markedly improved efficiencies compared to single agent anti-HER2 therapies. Trastuzumab, the first widely used anti-HER2 therapy, is a monoclonal antibody that binds to the extracellular domain of the HER2 receptor, which inhibits its dimerization with other members of the ERBB receptor family, thereby inhibiting downstream pathway activation. Several drugs investigated as potential therapeutic partners with trastuzumab in the treatment of HER2 positive cancers include: pertuzumab, which also inhibits dimerization of HER2 receptors; lapatinib, which blocks the tyrosine kinase domain of HER2 (as well as EGFR); deruxtecan, a topoisomerase I inhibitor; and tucatinib, an oral tyrosine kinase inhibitor that is highly selective for HER2.5-7,12,13
HER2/ERBB2 Overexpression in CRC: CRC HER2/ERBB2 overexpression is associated with poor patient outcomes compared to HER2 negative CRCs, similar to gastric and breast cancer.5 HER2 positive CRCs have an increased risk of dissemination to lungs, liver, central nervous system (similar to breast cancer), and peritoneum/ovaries.7,14 For unknown reasons, HER2 positive CRCs are found more commonly in left-sided and rectal cancers.7,15
The Cancer Genome Atlas Program (TCGA) identified amplification of the ERBB2 gene in 4% of CRCs.16 In a study of over 3000 CRCs, Richman et al found that 2.2% of Stage IV and 1.3% of Stage II – III CRCs demonstrated ERBB2 overexpression—the vast majority secondary to gene amplification. Furthermore, ERBB2 overexpression is more common in tumors with KRAS/NRAS/BRAF wild-type status, occurring in approximately 2% – 5% of these cancers compared to 0.2% – 1.4% of CRCs with KRAS, NRAS, or BRAF mutations.17 Others demonstrated that 3% – 5% of all mCRCs show over expression of HER2, increasing to approximately 10% in KRAS wild-type mCRCs.6
A number of studies have investigated the use of dual agent anti-HER2 therapy in HER2 positive CRCs, demonstrating improved patient outcomes, particularly in patients with KRAS negative wild-type CRCs. Objective response rates have varied from 10% to 71% with reported median progression-free survival from 2.9 to 8.1 months.5 For example in the MyPathway trial, 18 of 57 patients with HER2 positive mCRC had an objective response (40% in KRAS wild type and 8% in KRAS mutated cancers).18 The recently published MOUNTAINEER study of dual agent anti-HER2 therapy in chemotherapy-refractive HER2 positive RAS wild-type mCRC reported that in a cohort of 84 patients, 38% had an objective treatment response—one complete response and 29 with a partial response.6 Based on these results, the FDA granted accelerated approval to tucatinib, in combination with trastuzumab, for RAS wild type, HER2 positive unresectable or metastatic colorectal cancer that has progressed following fluoropyrimidine, oxaliplatin, and irinotecan based chemotherapy.19
These results have led to widespread requests to pathology departments to develop programs to help identify HER2 positive CRCs, which present significant challenges given the relatively low incidence of these cancers and the relative lack of experience in HER2 assessments in CRCs compared to breast and upper gastrointestinal cancer.
Identification of HER2 Positive Colorectal Cancers: Given the relatively recent adoption of anti-HER2 therapy in CRC, it is not surprising that criteria for diagnosis of HER2 positive CRCs are not standardized.
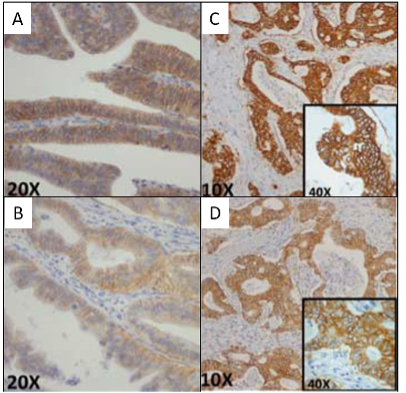
Immunohistochemistry (IHC) is the most commonly utilized method of identifying HER2 positive CRCs. In the HERACLES trial of mCRC patients, staining was classified from 0 to 3+ with higher thresholds than the accepted breast and upper gastrointestinal cancer protocols (3+ membranous HER2 staining in ≥ 50% of cells), equivocal (3+ membranous staining in > 10% but < 50% of cells, or cases with 2+ membranous staining in ≥ 50% of cells), or negative (absent staining or some HER2 staining not meeting criteria above) (Table 1 and Figure 2).20 Other potential diagnostic criteria were recently proposed based on breast and gastric cancer criteria, allowing for a HER2 positive diagnosis based on lower percentage staining of cancer cells.21
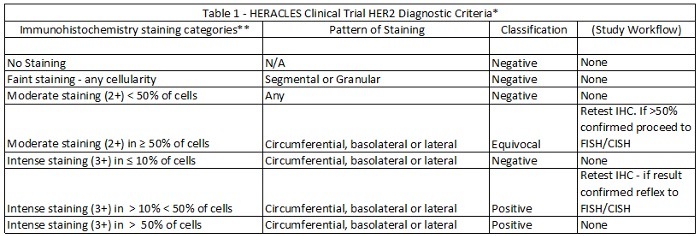
*Modified and used with permission from Valtorta et al.22
**Results based on use of the Ventana 4B5 antibody.

Figure 2: HERACLES Trial. Initial Immunohistochemistry scoring for HER2 in colon cancer. A – VENTANA and B – HercepTest: Weak to moderate staining; C – VENTANA and D – HercepTest: Intense staining. All circumferential, basolateral, or lateral pattern of staining. See text and Table 1 for final cutoffs. Modified and used with permission from Valtorta et al.22
ERBB2 amplification in the HERACLES trial of KRAS wild-type mCRCs using fluorescence (or silver) in situ hybridization (FISH) was considered positive with a ERBB2/CEN17 ratio ≥ 2.0,22 (CEP17 is a standard chromosome centromere control); similar to esophagogastric and breast cancers.23,24
Given the relative rarity of HER2 positive CRCs, detection of ERBB2 amplification via next generation sequencing (NGS) is a potential cost-effective way to identify these patients whose cancers are undergoing routine sequencing. One study demonstrated a good correlation between NGS derived ERBB2 amplification (CNV ≥ 6) and the IHC HERACLES scoring system.25 However as other studies have suggested differing cutoffs to predict likelihood of an anti-HER2 therapeutic effect, further research in this field is warranted.26
Circulating tumor DNA (ctDNA) is a potentially attractive method to identify tumor response to therapy as well as development of cancer resistance. Using serial blood samples from enrolled patients, the HERACLES trial identified increased ERBB2 alleles, as well as emergence of oncogenic activation of the MAPK and PI3K-AKT pathways (e.g., KRAS mutations, plus abnormalities of BRAF, ERBB2, EGFR, PIK3CA and PTEN) in the majority of patients on therapy over time. Emergence of these known and putative markers causing anti-HER2 pathway resistance and cancer progression was noted.27
Recognizing the relative uniqueness of individual cancers, many with inherent therapeutic susceptibilities defined by post-diagnostic molecular classifications, is a hallmark of the current era of precision oncology diagnostics. The recognition that anti-HER2 therapies can improve outcomes in a subset of patients with mCRC based on classification via advanced tissue testing represents a familiar pattern of progress for pathologists. Incorporating HER2 testing of colorectal cancers into pathology workflows is increasing in importance. This is due to increased knowledge of the pathophysiology of the cellular pathways linked to HER2 activation, significant advances in positive clinical trials of anti-HER2 therapies in colon cancer patients, and recent groundbreaking FDA approval for a combination anti-HER2 therapy.
As with other precision medicine diagnostics, collaboration with medical oncologists in devising specimen workflows, including institutional reflex testing programs based on cancer diagnosis and stage; standardized reporting formats and expected turnaround times are vital for optimizing patient care as many of these patients will have exhausted all standard therapies prior to exploring anti-HER2 options.
Undoubtedly, as clinical and translational research continues, further advances to better stratify patients whose cancers are susceptible to anti-HER2 therapy, including pathology and medical oncology society guidelines, will emerge. Nonetheless, using standard technologies and procedures, most pathology departments are positioned to incorporate HER2 testing in response to this important new and exciting development in cancer diagnostics and therapeutics.
References
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254.
- International Agency for Research on Cancer. Globocan: cancer fact sheets—colorectal cancer. 2021. Accessed April 17, 2024. https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-factsheet.pdf
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386-1422.
- Zeineddine FA, Zeineddine MA, Yousef A, et al. Survival improvement for patients with metastatic colorectal cancer over twenty years. NPJ Precis Oncol. 2023;7(1):16.
- Strickler JH, Yoshino T, Graham RP, Siena S, Bekaii-Saab T. Diagnosis and Treatment of ERBB2-Positive Metastatic Colorectal Cancer: A Review. JAMA Oncol. 2022;8(5):760-769.
- Strickler JH, Cercek A, Siena S, et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol. 2023;24(5):496-508.
- Sartore-Bianchi A, Amatu A, Porcu L, et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist. 2019;24(10):1395-1402.
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341-354.
- Uchida S, Sugino T. ERBB2-mutant gastrointestinal tumors represent heterogeneous molecular biology, particularly in microsatellite instability, tumor mutation burden, and co-mutated genes: an in silico study. Curr Issues Mol Biol. 2023;45(9):7404-7416.
- Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3(99):99ra86.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697.
- Tosi F, Sartore-Bianchi A, Lonardi S, et al. Long-term clinical outcome of trastuzumab and lapatinib for HER2-positive metastatic colorectal cancer. Clin Colorectal Cancer. 2020;19(4):256-62e2.
- Siena S, Di Bartolomeo M, Raghav K, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22(6):779-789.
- Sartore-Bianchi A, Lonardi S, Aglietta M, Martino C, Ciardiello F, Marsoni S, et al. Central nervous system as possible site of relapse in ERBB2-positive metastatic colorectal cancer: long-term results of treatment with trastuzumab and lapatinib. JAMA Oncol. 2020;6(6):927-929.
- Salem ME, Weinberg BA, Xiu J, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8(49):86356-86368.
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330-337.
- Richman SD, Southward K, Chambers P, et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238(4):562-570.
- Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20(4):518-530.
- Food and Drug Administration. FDA grants accelerated approval to tucatinib with trastuzumab for colorectal cancer. 2023. Accessed April 17, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tucatinib-trastuzumab-colorectal-cancer
- Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738-746.
- Fujii S, Magliocco AM, Kim J,et al. International harmonization of provisional diagnostic criteria for ERBB2-amplified metastatic colorectal cancer allowing for screening by next-generation sequencing panel. JCO Precis Oncol. 2020;4:6-19.
- Valtorta E, Martino C, Sartore-Bianchi A, et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015;28(11):1481-1491.
- Bartley AN, Washington MK, Ventura CB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016;140(12):1345-1363.
- Wolff AC, Somerfield MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2023;147(9):993-1000.
- Cenaj O, Ligon AH, Hornick JL, Sholl LM. Detection of ERBB2 amplification by next-generation sequencing predicts HER2 expression in colorectal carcinoma. Am J Clin Pathol. 2019;152(1):97-108.
- Torres-Jimenez J, Esteban-Villarrubia J, Ferreiro-Monteagudo R. Precision medicine in metastatic colorectal cancer: targeting ERBB2 (HER-2) oncogene. Cancers (Basel). 2022;14(15):3718.
- Siravegna G, Lazzari L, Crisafulli G, et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell. 2018;34(1):148-162 e7.

Joseph E. Willis, MD, FCAP, is a gastrointestinal pathologist in Cleveland, Ohio, and is a professor and vice chair for Translational Research in the Department of Pathology at Case Western Reserve University and University Hospitals Cleveland Medical Center. He received his medical degree from University College Cork Faculty of Medicine and is a fellow of the Royal College of Surgeons in Ireland.