- Home
- Member Resources
- Articles
- Doing a Lot with a Little: Molecular Testing on Cytology Specimens
The advances in radiographic techniques that enable precise targeting of lesions has revived attention to cytology specimens acquired by fine needle aspiration (FNA). One of the major advantages of FNA is that it is less invasive and better tolerated than a larger tissue biopsy and in many clinical scenarios provides sufficient material not only for morphological evaluation but also for ancillary studies including molecular testing. Accordingly, it is important to understand the pros and cons of molecular tests performed on cytological preparations.
What is special about molecular tests on cytology preparations compared to surgical pathology specimens
While there are a few methods specifically optimized for cytology samples, many of the current standard clinical molecular assays were initially designed and validated for surgical pathology preparations (i.e., formalin fixed paraffin embedded tissue blocks), including most fluorescent in situ hybridization (FISH) tests and next generation sequencing (NGS) panels. These latter tests therefore may require additional validation for their application to cytological preparations.
Nevertheless, technically most molecular studies can be performed on cytology specimens. While most of the cytological preparations, including FNAs, typically provide much smaller tissue samples which may be quantitatively insufficient for molecular analysis, the risk can be mitigated by increasing the number of needle passes (sampling) and using rapid on-site evaluation (ROSE). ROSE is a quality check unique to cytology where the cytologist performing the FNA is present with the patient and can directly confirm adequacy in a way that is not possible in surgical biopsies (frozen sections not being quite as immediate or directly patient-facing). ROSE also enables specimen triaging and up-front decision-making about (additional) passes specifically destined for molecular testing based on the initial diagnostic impression. Since the cytology procedure often precedes the surgical biopsy, utilization of the cytology specimen can dramatically shorten the time to molecular result.
Adequacy is the single most important parameter of a cytological specimen being considered for molecular test – the quantity of the evaluable tumor nucleic acid in the tested material. Molecular studies need an absolute cell count to ensure adequacy. Each institution establishes its cut-off to ensure adequacy which typically ranges 500-1000 cells on cell block sections.1-3 Usually if adequate quantity is provided, the quality of such specimens is at least similar to, if not superior to, that of a surgical pathology preparation.3,4 The morphologic indicator of the material quantity is the relative tumor percentage which can be often estimated on-site. The cytopathologist evaluates and provides the overall cellularity and fraction of tumor cells in the sample. This is especially important for molecular techniques that are sensitive to the neoplastic percentage, e.g., microarray and NGS platforms which usually require at least 5-10% of tumor cellularity.
Finally, cytology specimens have the advantage of faster fixation (or no fixation at all) and the use of milder fixatives (e.g., alcohols) compared to formalin, leading to better quality nucleic acids. Formalin fixation has known negative effects on DNA quality e.g. nucleic acid fragmentation, smaller library insert sizes, greater coverage variability, and an increase in C to T transitions (especially at CpG dinucleotides).5 However, for typical cytology smear fixation, 95% ethanol dipping solution and 95% isopropanol spray are frequently used, and less commonly methanol. Both alcohol-fixed, Pap-stained, and air-dried, DQ-stained smears are acceptable for molecular studies.6-8 Premanufactured solutions for liquid-based preparations are either methanol-based or contain low amounts of formaldehyde in their formulation; somewhat lower quality DNA was found in material preserved in formaldehyde-based solutions.9 When considering using smears for a molecular test, xylene-based mounting media should be avoided as it results in lower DNA yield.6 For cell block (CB) preparations, fixation with 10% buffered formalin with avoidance of Bouin’s solution or any fixative containing heavy metals is recommended. Methanol is used in some CB preparations which is not a standard fixative typically validated for molecular studies; CBs from such preparations can be post-fixed in formalin to avoid re-validation for immunohistochemistry and NGS studies.10

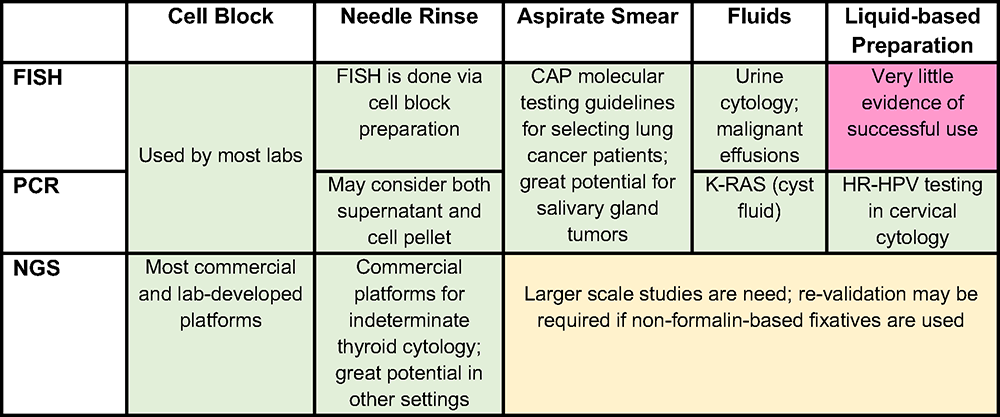
Figure 1. Green – either a validated commercial assay, multicenter studies and/or evidence-based recommendations by a pathology or clinical society exists; Yellow – evidence is provided by multiple studies, require more studies; Red – little (single or few studies) evidence provided
Abbreviations: FISH = fluorescence in-situ hybridization; PCR = polymerase chain reaction; NGS = next generation sequencing
What are the pros and cons of different cytological preps for molecular tests
To provide the best material for molecular tests, the correct choice between different cytological preparations from the same sample should be considered (Figure 1). Multiple preparations from the same lesion often co-exist. Cytological preparations that can be used for molecular studies include cell blocks, needle rinses, direct smears, fluid (effusion, cyst fluid, etc.) and liquid-based preparations. According to CAP guidelines for evaluation of lung cancer, any cytology sample (including aspirate smears) with adequate cellularity and preservation may be tested.11
Cell blocks (CB) are traditionally used for molecular diagnostic tests because they closely recapitulate formalin fixed paraffin embedded (FFPE) surgical pathology specimens and usually do not require additional validation (except for methanol-based preparations, discussed above). The major shortcoming of the CB is that tumor cells or tissue particles are often widely spaced, resulting in low tumor cellularity per section area. To increase nucleic acid yield, cell/tissue concentration approaches for CB preparation (e.g., collodion bag and a variety of commercial preparations) can be used.10,12 Simply providing more sections than would be used for a surgical specimen is another option. Tumor percentage can be increased by macrodissecting the regions of highest neoplastic cellularity.1
Needle rinses are another common preparation submitted for molecular test. This specimen type can potentially provide higher yield of nucleic acids than the CB sections if the CB is unavailable, CB tumor percentage is too low, and/or if all prepared slides must be retained to comply with slide retention policies (see additional comments below on slide retention).13,14 Both cell pellets and supernatant from the needle rinses can be used. The supernatant is an underrecognized “gold mine” for DNA and RNA, especially in limited specimens that require both molecular testing and a tissue diagnosis (which is done on the pellet converted to a CB), since the supernatant is typically discarded.15 The disadvantages of the supernatant use for molecular studies are difficulty to estimate tumor fraction in a sample and inability to perform sample enrichment (i.e. microdissection in the CB sections). If sample stability is an issue (longer time to testing), fixatives13,14 or preservative tubes16 may be used to preserve the nucleic acids. Additional validation for samples rinsed into solutions with no fixative (e.g., HBSS, RPMI) or methanol-based fixatives may be required.
Using material directly from cytology smears for molecular tests is attractive because of the obvious advantage of getting an excellent quality material with higher nucleic acid yield than from the CBs.3,17 Aspirate smears used for molecular tests can be air-dried or alcohol fixed. When limited material is available, de-stained smears (both from Pap and Diff-Quick stain) are safe for use with minimal loss of DNA quality.18 CAP guidelines allow for sacrificing diagnostic material if it is medically necessary; the problem of sacrificing diagnostic slides can be mitigated by digital scanning of the slides before scraping them.18
Fluids can be a great source of material for molecular testing: both cellular content as well as cell free DNA (especially in cyst fluids) are used. However, as the fluids are not fixed, nucleic acid degradation or poor cellular preservation may be an issue. To minimize this issue, rapid timing from sampling to test and/or use of equal volume of alcohol based collection media and/or storage in a tube designed for the preservation of nucleic acids should be considered.19
Finally, liquid-based preparations are a great source of material for PCR-based techniques, particularly high-risk HPV testing. Nucleic acid can be extracted both from rinse solutions and cells scraped off the sides. Studies showed that the results of NGS from liquid-based preparations are at least comparable to direct smears and core biopsies. Moreover, increased values for the mean insert size, mean target coverage, and percent usable bases, along with a lower duplication rate were found in smears and liquid-based preparation, suggesting even higher quality DNA than FFPE, but more data is needed to support their broader use.3
What types of molecular tests can be used on cytology specimens
While some of the commercial FISH-based platforms performed on cytology specimens are broadly used in clinical practice (e.g., in urine cytology), others require additional validation and comparison with surgical preparations. Among those, some of the promising areas are lung and salivary gland cytopathology.11,20 FISH for ALK or ROS1 rearrangements in NSCLC showed results acceptable for clinical use, both on diagnostic smears and material from metastatic effusions.6,21 In some clinical scenarios, a metastatic effusion might be the only source of diagnostic material; in this context, the concordance rate between FISH and immunohistochemical stains for ALK or ROS1 on pleural effusion cell blocks was 100%.21 In one study, FISH for MECT1/MAML2 gene fusion and rearrangements of PLAG1, MYB, or ETV6, performed on salivary gland FNA smears approached a specificity of 100% and a sensitivity of 66.7% (overall sensitivity 93.3% for combined FISH and cytological analyses) with the conclusion that a diagnosis from a frozen section for the extent of surgery might not be necessary when the FISH analysis is positive.20
Cytology specimens are excellent for PCR-based tests, including those for single gene mutations (KRAS in pancreatic cyst fluid, BRAF for thyroid cancers, etc.) or fusion products (BCR::ABL1) as they are usually highly sensitive and do not require significant amounts of tested nucleic acid. Liquid-based preparations are the preferable modality for screening cervical cytology and monitoring high-risk HPV infection; they are either PCR-based or use nucleic acid transcription-mediated amplification.
The advantages of using cytology preps for NGS include quicker fixation or, if the platform is validated, minimal/no fixation at all, improving the quality of the input nucleic acids. In case both surgical and cytology samples from the same lesion are available, the preference usually goes to preparation with the highest amount of tumor cells and side by side comparison of both samples is highly encouraged. While many laboratory-developed NGS panels are available for use, there are also specific panels developed for cytology specimens. Indeterminate results of thyroid cytology can be resolved by a molecular analysis via commercially available NGS platforms that use proprietary genomic sequencing classifiers, targeting different classes of molecular alterations, including point mutations, insertions/deletions, gene fusions, copy number alterations, and gene expression alterations by sequencing both DNA and RNA.22,23 Both thyroid NGS commercial platforms require a significant amount of material (at least 2-4 dedicated needle passes) for adequate testing quality and showed comparable good diagnostic performance.22,23,24
In conclusion, molecular testing on cytology preparations is a relatively new and rapidly developing field with great potential. While well-established commercial molecular platforms as well as laboratory-developed testing for cytological specimens exist, others require a certain level of caution before submitting cytology samples for molecular testing. At minimum confirmation of validation for cytology preparations and close check of quantity and quality of submitted material is expected.
References:
- Roy-Chowdhuri S, Stewart J. Preanalytic Variables in Cytology: Lessons Learned From Next-Generation Sequencing-The MD Anderson Experience. Arch Pathol Lab Med. 11 2016;140(11):1191-1199.
- Kalhor N, Wistuba II. Perfecting the fine-needle aspirate cell block. Cancer Cytopathol. Mar 2013;121(3):109-10.
- Hwang DH, Garcia EP, Ducar MD, Cibas ES, Sholl LM. Next-generation sequencing of cytologic preparations: An analysis of quality metrics. Cancer Cytopathol. Oct 2017;125(10):786-794.
- Treece AL, Montgomery ND, Patel NM, et al. FNA smears as a potential source of DNA for targeted next-generation sequencing of lung adenocarcinomas. Cancer Cytopathol. Jun 2016;124(6):406-14.
- Spencer DH, Sehn JK, Abel HJ, Watson MA, Pfeifer JD, Duncavage EJ. Comparison of clinical targeted next-generation sequence data from formalin-fixed and fresh-frozen tissue specimens. J Mol Diagn. Sep 2013;15(5):623-33.
- Dejmek A, Zendehrokh N, Tomaszewska M, Edsjö A. Preparation of DNA from cytological material: effects of fixation, staining, and mounting medium on DNA yield and quality. Cancer Cytopathol. Jul 2013;121(7):344-53.
- Killian JK, Walker RL, Suuriniemi M, et al. Archival fine-needle aspiration cytopathology (FNAC) samples: untapped resource for clinical molecular profiling. J Mol Diagn. Nov 2010;12(6):739-45.
- Sorber L, Claes B, Zwaenepoel K, et al. Evaluation of Cytologic Sample Preparations for Compatibility With Nucleic Acid Analysis. Am J Clin Pathol. Feb 03 2022;157(2):293-304.
- Fujii T, Asano A, Shimada K, Tatsumi Y, Obayashi C, Konishi N. Evaluation of RNA and DNA extraction from liquid-based cytology specimens. Diagn Cytopathol. Oct 2016;44(10):833-40.
- Shidham VB. CellBlockistry: Chemistry and art of cell-block making - A detailed review of various historical options with recent advances. Cytojournal. 2019;16:12.
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. Mar 2018;142(3):321-346.
- Rollins S, Russell D. Cytopathology in Focus: Cell Blocks: Getting the most from the least invasive method. CAP Today; 2017.
- Zhou H, Mody DR, Smith D, et al. FNA needle rinses preserved in Cytolyt are acceptable specimen type for mutation testing of thyroid nodules. J Am Soc Cytopathol. 2015 May - Jun 2015;4(3):128-135.
- Krane JF, Cibas ES, Alexander EK, Paschke R, Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. Jun 2015;123(6):356-61.
- Roy-Chowdhuri S, Mehrotra M, Bolivar AM, et al. Salvaging the supernatant: next generation cytopathology for solid tumor mutation profiling. Mod Pathol. 07 2018;31(7):1036-1045.
- Fuller MY, Mody D, Hull A, Pepper K, Hendrickson H, Olsen R. Next-Generation Sequencing Identifies Gene Mutations That Are Predictive of Malignancy in Residual Needle Rinses Collected From Fine-Needle Aspirations of Thyroid Nodules. Arch Pathol Lab Med. Feb 2018;142(2):178-183.
- Harada S, Agosto-Arroyo E, Levesque JA, et al. Poor cell block adequacy rate for molecular testing improved with the addition of Diff-Quik-stained smears: Need for better cell block processing. Cancer Cytopathol. Aug 2015;123(8):480-7.
- Huang M, Wei S. Overview of Molecular Testing of Cytology Specimens. Acta Cytol. 2020;64(1-2):136-146.
- Michael CW, Davidson B. Pre-analytical issues in effusion cytology. Pleura Peritoneum. Mar 01 2016;1(1):45-56.
- Evrard SM, Meilleroux J, Daniel G, et al. Use of fluorescent in-situ hybridisation in salivary gland cytology: A powerful diagnostic tool. Cytopathology. Aug 2017;28(4):312-320.
- Wang W, Tang Y, Li J, Jiang L, Jiang Y, Su X. Detection of ALK rearrangements in malignant pleural effusion cell blocks from patients with advanced non-small cell lung cancer: a comparison of Ventana immunohistochemistry and fluorescence in situ hybridization. Cancer Cytopathol. Feb 2015;123(2):117-22
- Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 04 15 2018;124(8):1682-1690.
- Angell TE, Wirth LJ, Cabanillas ME, et al. Analytical and Clinical Validation of Expressed Variants and Fusions From the Whole Transcriptome of Thyroid FNA Samples. Front Endocrinol (Lausanne). 2019;10:612.
- Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol. 01 01 2021;7(1):70-77.

Vladislav V. Makarenko, MD, PhD, is a Fellow in Hematopathology at Brigham and Women's Hospital. He also completed a fellowship in Cytology at Massachusetts General Hospital.

Annette S. Kim, MD, PhD, FCAP is the Henry Clay Bryant Professor and Division Head of Diagnostic Genetics and Genomics at the University of Michigan. Dr. Kim’s research program has focused on the study of hematolymphoid malignancies, including miRNAs in myelodysplastic syndromes, myeloid and lymphoid mutational patterns, and test utilization management. She has served as a member of Molecular Oncology Committee and is currently Vice-Chair of the Personized Healthcare Committee for the College of American Pathologists. She is the Vice-Chair of the ASH Precision Medicine committee. In 2019, Dr. Kim was awarded the CAP Public Service Award.