- Home
- Member Resources
- Articles
- BCL2-Rearrangement Negative Follicular Lymphomas: Oh No, Say It Ain’t So!
Follicular lymphoma (FL) is the third-most-common lymphoma in the United States (after diffuse large B cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma).1 FL arises from germinal center B cells, as marked by the typical expression of CD10 and BCL6, with histologic evidence of follicular architecture in the majority of cases. In 85% - 90% of FL cases, there is a pathogenic t(14;18)(q32;q21)/IGH::BCL2 rearrangement (BCL2-R) which results in the overexpression of BCL2. Co-expression of BCL2 with a germinal center marker such as CD10 by immunohistochemical staining (IHC) is generally considered diagnostic of conventional follicular lymphoma (cFL) with supporting histomorphologic and architectural findings.2–4 The absence of BCL2 expression may present a diagnostic challenge, warranting further characterization by immunohistochemistry and/or molecular study (Figure 1).
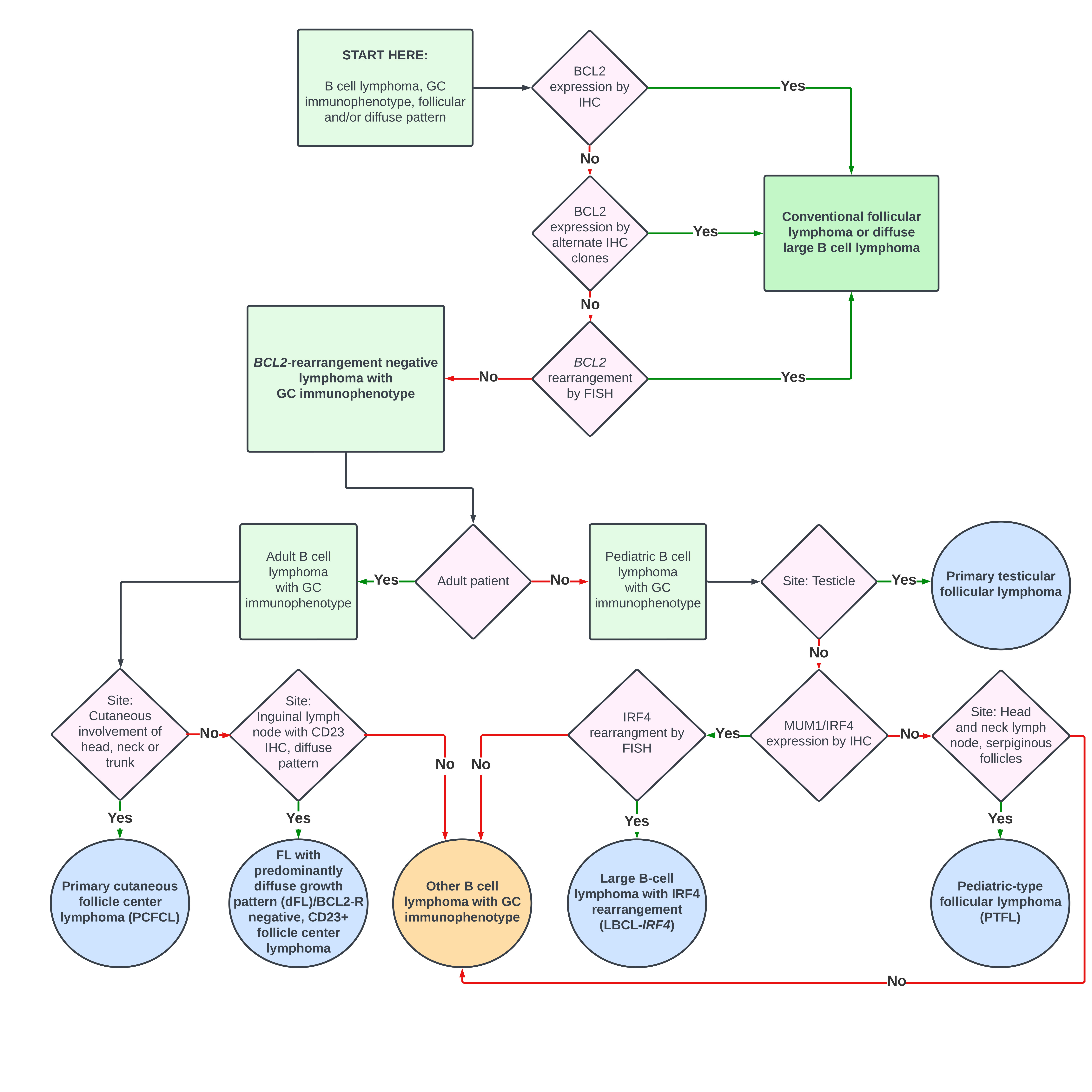
False negativity for BCL2 by IHC may occur when activation-induced cytidine deaminase (AID)-associated mutagenesis targets the BCL2 locus.5,6 Antibodies targeting other epitopes routinely identify these variants, which account for about half of alleged BCL2-negative FL. Therefore, multiple antibody clones should be attempted, if feasible, before determination that a FL is BCL2 negative.7
Three different BCL2 antibody variants (clones 100/D5, E17, SP66) are available for assessing BCL2 expression. The standard BCL2 antibody (clone 100/D5) targets an epitope encompassing codons 41 - 54 of the BCL2 protein and may yield negative results in instances involving small in-frame deletions and missense mutations within the targeted epitope.8 Alternative BCL2 antibody clones, targeting different segments of the BCL2 protein, are also available. Clones E17 and SP66 have demonstrated positivity in cases where the standard BCL2 clone is negative. Specifically, clone E17 targets amino acids 61 - 76 of the BCL2 protein, while SP66 targets the N-terminal region of the BCL2 protein.7 Conversely, positive BCL2 IHC can also occur in the absence of a BCL2-R due to transcriptionally or translationally regulated overexpression through a variety of mechanisms in several FL variants.
The workup of any BCL2-R-negative case of FL by IHC should be confirmed by FISH. However, FISH can also be falsely negative due to the design of the particular IGH::BCL2 probes that are used. Each commercially available probe targets different regions of BCL2. Depending upon the genomic location of the probes, there may still be a split BCL2 signal without IGH::BCL2 fusion, or a fusion may not be detected.
Once the diagnosis of a bona fide BCL2-R negative FL is made, the diagnostic subcategorization can be determined by additional factors including age, site, histologic pattern, immunophenotype, and genotype (Table 1, Figure 1) with clues to the diagnostic category often incorporated into the name of the entity.2,9,10 Pediatric-type follicular lymphoma (PTFL), primary testicular follicular lymphoma, and large B-cell lymphoma with IRF4 rearrangement (LBCL-IRF4) are more common in the pediatric population. These subtypes are also seen in adults, especially young adults.2,11 By contrast, adults are more commonly affected by primary cutaneous follicle center lymphoma (PCFCL) and follicular lymphoma with predominantly diffuse growth pattern (dFL),2 a category largely overlapping with BCL2-R negative, CD23+ follicular center lymphoma.9,10,12
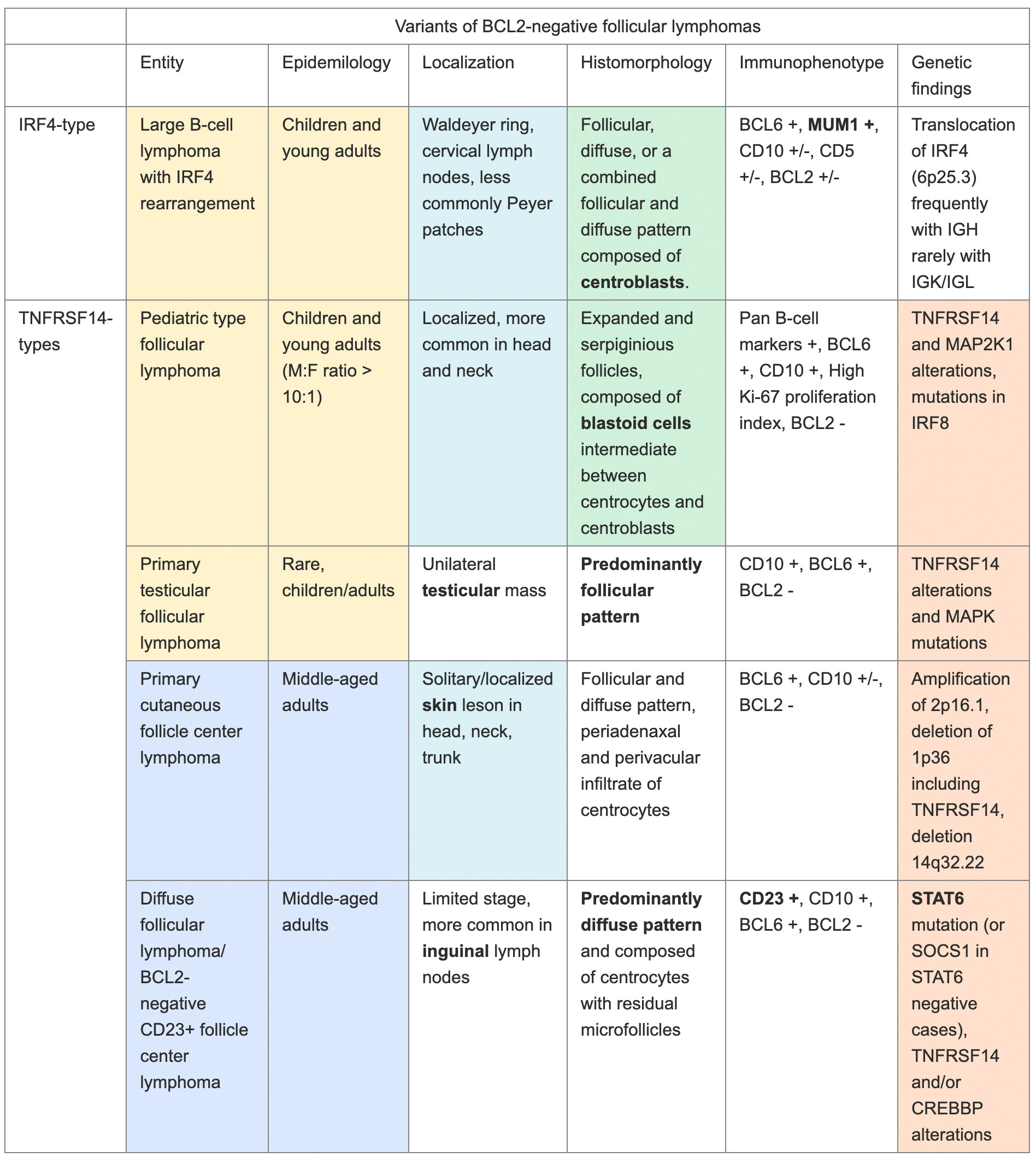
As indicated in many of the diagnostic names, the site of disease is critical, as several of the BCL2-R negative FLs present in a specific anatomic location. PTFL is most common in the head and neck, particularly Waldeyer’s ring, while primary testicular follicular lymphoma typically presents as a unilateral testicular mass. dFL most commonly presents in inguinal lymph nodes (other sides also possible but less common); and PCFCL presents in the skin, particularly in the upper portions of the body. Finally, LBCL-IRF4 is the only BCL2-R negative FL with no site predilection, although most cases are found in the head and neck, with fewer in the GI tract.
Most BCL2-R negative FLs demonstrate either follicular or diffuse histologic patterns. However, as indicated by the descriptive name, dFL is predominantly diffuse with only remnant follicles (marked by CD21 and CD35), although there is diffuse CD23 positivity on neoplastic B cells. Both LBCL-IRF4 and PTFL may present with higher grade disease (increased centroblasts) and should not be mistaken for aggressive large cell lymphomas, such as high grade cFL or even diffuse large B cell lymphoma if the histology is diffuse or unclear (such as in a small biopsy).
BCL2-R negative cases tend to be more genomically stable than cFL. Recurrent mutations seen in cFL, such as the histone methyltransferases KMT2D and EZH2, the histone acetyltransferases CREBBP, EP300, and MEF2B, and the tumor necrosis factor receptor TNFRSF14, can also occur in BCL2-R negative cases but generally at much lower frequency. The exception is TNFRSF14, which is relatively more common in many diagnostic entities that are BCL2-R negative. Similarly, cFL are also characterized by a wide range of recurrent gains in the chromosomal regions 1q, 2p15, 7, 8q, 12q, 18p, and 18q, as well as losses in 1p36, 3q, 6q, 9p, 10q, 11q, 13q, and 17p.2,3,12 By contrast, BCL2-R negative follicular lymphomas display fewer copy number alterations. In BCL2-R negative FL, ABC-like and post-GCB signatures are found to be overexpressed with evidence of ongoing somatic hypermutation, a feature of GCB-cells. Therefore, it is suggested that a subset of BCL2-R negative follicular lymphoma has a late GCB cell phenotype.13
Based on the genomics, BCL2-R negative FLs can be subdivided into the IRF4-type FLs (Table 1, top entry) and the TNFSRF14-type FLs (Table 1, bottom four entries highlighted in peach). As the name indicates, LBCL-IRF4 is characterized by translocations of IRF4 (IRF4-R), corresponding to the atypical strong MUM1 positivity of these cases. However, this entity should not be confused with the secondary acquisition (progression event) of an IRF4-R, as the more favorable prognosis is associated solely with LBCL-IRF4.
TNFSRF14-type FLs have recurrent mutations or deletions (1p36) of TNFSRF14 or in the downstream MAPK signaling pathway. The exception to this rule is dFL with highly recurrent mutations in STAT6, or in the upstream regulator of STAT6 encoded by SOC1 in STAT6-negative cases. TNFSRF14 mutations are less common in dFL. Although TNFSRF14 mutations and deletions are also seen in cFL, these lesions are less common than the recurrent cFL mutations in epigenetic genes.2,3
The importance of discriminating a true BCL2-negative FL from cFL is underscored by their differences in treatment and prognosis. Conservative management yields a good prognosis in limited stage PTFL, LBCL-IRF4, PCFCL, and dFL disease. Primary testicular FL is too rare for robust prognostic studies, but one meta-analysis also suggests excellent outcomes after localized resection with or without chemotherapy. As these entities represent potential pitfalls to the practicing pathologist, further education and recognition of these diagnoses is required for an appropriately targeted workup and precise classification.
References
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. 2010. https://seer.cancer.gov/csr/1975_2017/
- WHO Classification of Tumours Editorial Board, ed. Haematolymphoid Tumours [Internet; Beta Version Ahead of Print]. Vol 11. 5th ed. International Agency for Research on Cancer; 2022.
- Nann D, Ramis-Zaldivar JE, Müller I, et al. Follicular lymphoma t(14;18)-negative is genetically a heterogeneous disease. Blood Adv. 2020;4(22):5652-5665. doi:10.1182/bloodadvances.2020002944
- Leich E, Salaverria I, Bea S, et al. Follicular lymphomas with and without translocation t(14;18) differ in gene expression profiles and genetic alterations. Blood. 2009;114(4):826-834. doi:10.1182/blood-2009-01-198580
- Casellas R, Basu U, Yewdell WT, Chaudhuri J, Robbiani DF, Di Noia JM. Mutations, kataegis and translocations in B cells: Understanding AID promiscuous activity. Nat Rev Immunol. 2016;16(3):164-176. doi:10.1038/nri.2016.2
- Chandra V, Bortnick A, Murre C. AID targeting: Old mysteries and new challenges. Trends Immunol. 2015;36(9):527-535. doi:10.1016/j.it.2015.07.003
- Adam P, Baumann R, Schmidt J, et al. The BCL2 E17 and SP66 antibodies discriminate 2 immunophenotypically and genetically distinct subgroups of conventionally BCL2-"negative" grade 1/2 follicular lymphomas. Hum Pathol. 2013;44(9):1817-1826. doi:10.1016/j.humpath.2013.02.004
- Masir N, Campbell LJ, Jones M, Mason DY. Pseudonegative BCL2 protein expression in a t(14;18) translocation positive lymphoma cell line: a need for an alternative BCL2 antibody. Pathology. 2010;42(3):212-216. doi:10.3109/00313021003631296
- Laurent C, Cook JR, Yoshino T, Quintanilla-Martinez L, Jaffe ES. Follicular lymphoma and marginal zone lymphoma: how many diseases? Virchows Archiv. 2023;482(1):149-162. doi:10.1007/s00428-022-03432-2
- Goodlad J, Cerroni L, Swerdlow S. Recent advances in cutaneous lymphoma—implications for current and future classifications. Virchows Archiv. 2023;482(1):281-298. doi:10.1007/s00428-022-03421-5
- Garrces S, Xu J, Li S. Primary testicular follicular lymphoma. Human Pathology Reports. 2022;27:300606. doi:10.1016/j.hpr.2022.300606
- Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009;113(5):1053-1061. doi:10.1182/blood-2008-07-168682
- Leich E, Zamo A, Horn H, et al. MicroRNA profiles of t(14;18)-negative follicular lymphoma support a late germinal center B-cell phenotype. Blood. 2011;118(20):5550-5558. doi:10.1182/blood-2011-06-361972

Annette S. Kim, MD, PhD, FCAP is the Henry Clay Bryant Professor and Division Head of Diagnostic Genetics and Genomics at the University of Michigan. Dr. Kim’s research program has focused on the study of hematolymphoid malignancies, including miRNAs in myelodysplastic syndromes, myeloid and lymphoid mutational patterns, and test utilization management. She has served as a member of Molecular Oncology Committee and is currently Vice-Chair of the Personized Healthcare Committee for the College of American Pathologists. She is the Vice-Chair of the ASH Precision Medicine committee. In 2019, Dr. Kim was awarded the CAP Public Service Award.

Mark Girton, MD, is a clinical assistant professor of pathology at the University of Michigan who practices hematopathology and clinical chemistry. He received his medical degree at Paul L. Foster School of Medicine, Texas Tech Health Sciences Center El Paso and completed residency and fellowship training at the University of Virginia. His interests include medical education, reactive and neoplastic lymphoid disease, hemoglobinopathy, quality improvement, and test utilization.

Anup Jnawali, MD, is currently a hematopathology fellow at University of Michigan. He completed his AP/CP residency from University of Massachusetts Chan Medical School. He is starting his GI/Liver pathology fellowship at Beth Israel Deaconess Medical Center. His area of interest includes lymphoid malignancies involving GI tract, digital pathology and implementation of AI and machine learning in pathology.