- Home
- Member Resources
- Articles
- Allogeneic Cellular Therapy – Diagnostic Challenges and Opportunities for Laboratory Practice
Allogeneic cell therapy (ACT) is an emerging therapeutic area with great potential to impact patients affected by cancer, infectious disease, and autoimmunity.1,2 Over 500 different ACTs are currently in preclinical or early phase clinical trials, with the number of therapeutic indications consistently increasing.3,4 Also called off-the-shelf cell therapies, ACTs have several potential advantages over autologous (ie, patient-derived) cell therapies. These include more standardized and scalable manufacturing, which may translate to lower production costs; improved product consistency; increased patient access; and reduced treatment delays and treatment failure, especially in multiply pretreated patients.5,6 However, ACTs also present challenges to patient and donor selection; compatibility; risk of allosensitization; safety/efficacy; and monitoring, some of which are unique to this type of therapy. To better understand the implications of emerging ACTs on laboratory practice, a joint working group with members from the College of American Pathologists Personalized Healthcare Committee (FL, GM, MA) and the Histocompatibility and Identity Testing Committee (MG, JZ) was established. Based on findings of a scoping literature review, this article provides a brief overview of the most significant challenges of ACT for laboratory medicine and pathology. It also highlights the areas of laboratory practice that may be most impacted by the need to develop and deploy appropriate biomarkers and diagnostic testing for this novel type of therapy.
What are the most common ACTs and indications relevant for diagnostic laboratory practice?
An overview of cell-based therapeutic approaches and strategies is provided in Table 1. ACTs are a particular type of adoptive cell therapy or immune effector cell therapy, a strategy where specific immune cells from a donor are collected, expanded, and engineered in vitro before being returned to a recipient’s body to eliminate pathogens and/or cancer cells.3,7 ACT is therefore distinct from allogeneic stem cell therapy, where the recipient’s hematopoietic system is regenerated or replaced by infusing hematopoietic stem cells from a healthy donor's bone marrow, peripheral blood, or umbilical cord blood to treat blood and immune disorders. ACT strategies include engineered T-cell receptor (TCR) T-cell therapy (in which T cells are engineered to recognize tumor antigens through specific T-cell receptors) and CAR-T cell therapy (in which T cells are genetically modified to express chimeric antigen receptors [CARs] that target specific tumor antigens).3 CAR-T cell therapy has become a cornerstone in the treatment of relapsed/refractory B-cell malignancies, and recent data has demonstrated efficacy for T-cell malignancies and solid tumors, such as pediatric neuroblastoma, synovial sarcoma, melanoma, and human papillomavirus-associated cancers.8–10 Other indications under investigation cover a range of noncancer conditions, including autoimmune conditions11, autoimmune rheumatic diseases12, and inducing tolerance in organ transplantation.13 Although many ACTs are derived from immune cell subsets (eg, NK, T cells, macrophages), other modified allogeneic cell types, such as mesenchymal stem cells and neuronal progenitors, are being investigated for treatment on autoimmune disease, neurodegenerative disorders, and other nonmalignant conditions.
Of note, CAR therapies have to date heavily relied on the use of autologous T cells. Allogeneic CAR-T therapy not only differs in the use of donor T cells (and other immune cell subsets, such as NK cells and macrophages) as the starting material, but also in the approaches to design and manufacturing.14,15 As described in a recent systematic review, strategies for allogeneic CAR cell production include additional genetic modifications and the selection of alternative cell sources or cell subpopulations, such as γδ T cells; induced pluripotent stem cells; umbilical cord blood T cells; memory T-cell subpopulations; virus-specific T cells; and cytokine-induced killer cells.5 Importantly, our literature search revealed that allogeneic CAR cell products differ widely in manufacturing complexity, resulting in differences in immunogenicity, immunophenotype, proliferation capacity, persistence in vivo, and efficacy, all of which pose challenges to developing standardized approaches for detection and monitoring of ACT products. Ongoing clinical studies are focused on improving allogeneic CAR cell product function and adaptation to additional disease contexts, but how these ACT products will impact the clinical laboratory remains relatively unexplored.
Table 1: Overview of cell-based therapeutic approaches and strategies.
| Type or concept of cell-based therapeutic approach/strategy | Explanation/Definition |
|---|---|
| Allogeneic stem cell therapy |
|
| Engineered cellular therapies |
|
| Adoptive cell therapy (ACT)/ Immune effector cell therapy (IECT) | A therapeutic approach in which specific immune cells are collected, expanded, and modified (if applicable) before being reinfused into a recipient to enhance immune responses against disease |
| Allogeneic cell therapy (ACT) | A subset of ACT in which immune cells used for therapy are derived from a donor, rather than the recipient, and therefore require immune compatibility considerations |
| T-cell receptor (TCR) T-cell therapy |
|
| Chimeric antigen receptor (CAR) T-cell therapy |
|
| Tumor-infiltrating lymphocyte (TIL) therapy |
|
| Macrophage-based cell therapy | Therapy using autologous or allogeneic macrophages, often engineered (eg, CAR-macrophages), to enhance tumor phagocytosis and modulate the immune microenvironment |
| Mesenchymal stem cell (MSC) therapy | Uses MSCs from bone marrow, adipose tissue, or umbilical cord for regenerative medicine and immune modulation in conditions such as graft-versus-host disease, autoimmune disorders, and tissue repair |
Challenges of ACTs
Based on the currently available literature, there are many potential challenges to the development and implementation of ACTs, ranging from manufacturing and regulatory aspects to patient care and treatment outcome (see Table 2).
Table 2: Overview of challenges in ACT
| Manufacturing and Scalability |
|
| Patient Selection and Compatibility |
|
| Risk of Allosensitization | ACTs may generate an immune response with these potential consequences:
|
| Safety Concerns |
|
| Efficacy Challenges |
|
| Monitoring and Long-term Follow-up |
|
Patient selection, donor compatibility, and safety/efficacy are predominantly affected by the immunologic disparity between donor and recipient, potentially leading to host-mediated immune rejection, graft-versus-host disease, and allosensitization.5 Allosensitization, where the patient's immune system becomes sensitized to the donor cells, poses a significant challenge, as it can potentially lead to reduced efficacy of the treatment over time, increased risk of rejection, and potential inability to receive future transplants or cell therapies. Pre-existing HLA donor-specific antibody, which is associated with graft failure and delayed engraftment in allogeneic stem cell transplantation, could similarly lead to rejection of allogeneic cellular products. An active area of preclinical research in ACT is the development of strategies to minimize immune rejection through manipulation of the HLA class I and HLA class II system to include targeted knock-out; constitutive expression of mutant fusion proteins; gene-editing technologies such as the CRISPR/Cas9 system; zinc finger nucleases; or transcription activator-like effector nucleases. Importantly, these approaches carry significant risks, such as off-target mutagenesis and other off-target effects, as well as gene rearrangements and chromosomal instability. Other approaches are aimed at the broader immune microenvironment to enhance antitumor immunity. They include engagement of other cell types; immune checkpoint inhibition using monoclonal antibodies; and modification of complex immune pathways and mechanisms through engineering and combination treatment approaches, all of which could affect tumor trafficking, tumor microenvironment composition, and immunophenotypes of cell subsets while potentially breaking peripheral tolerance and triggering autoimmune responses. Moreover, CAR-T cell therapy in particular is associated with a range of acute and subacute toxicities in up to 95% of patients, such as cytokine-release syndrome; immune effector cell-associated neurotoxicity syndrome; movement disorders; immune effector cell-associated hematotoxicity; immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome; risk of infection due to CAR-T cell-induced B-cell aplasia; and hypogammaglobulinaemia.9,16,17 Of note, laboratory testing plays an essential role in the diagnosis and monitoring of these toxicities and informs supportive care and management.
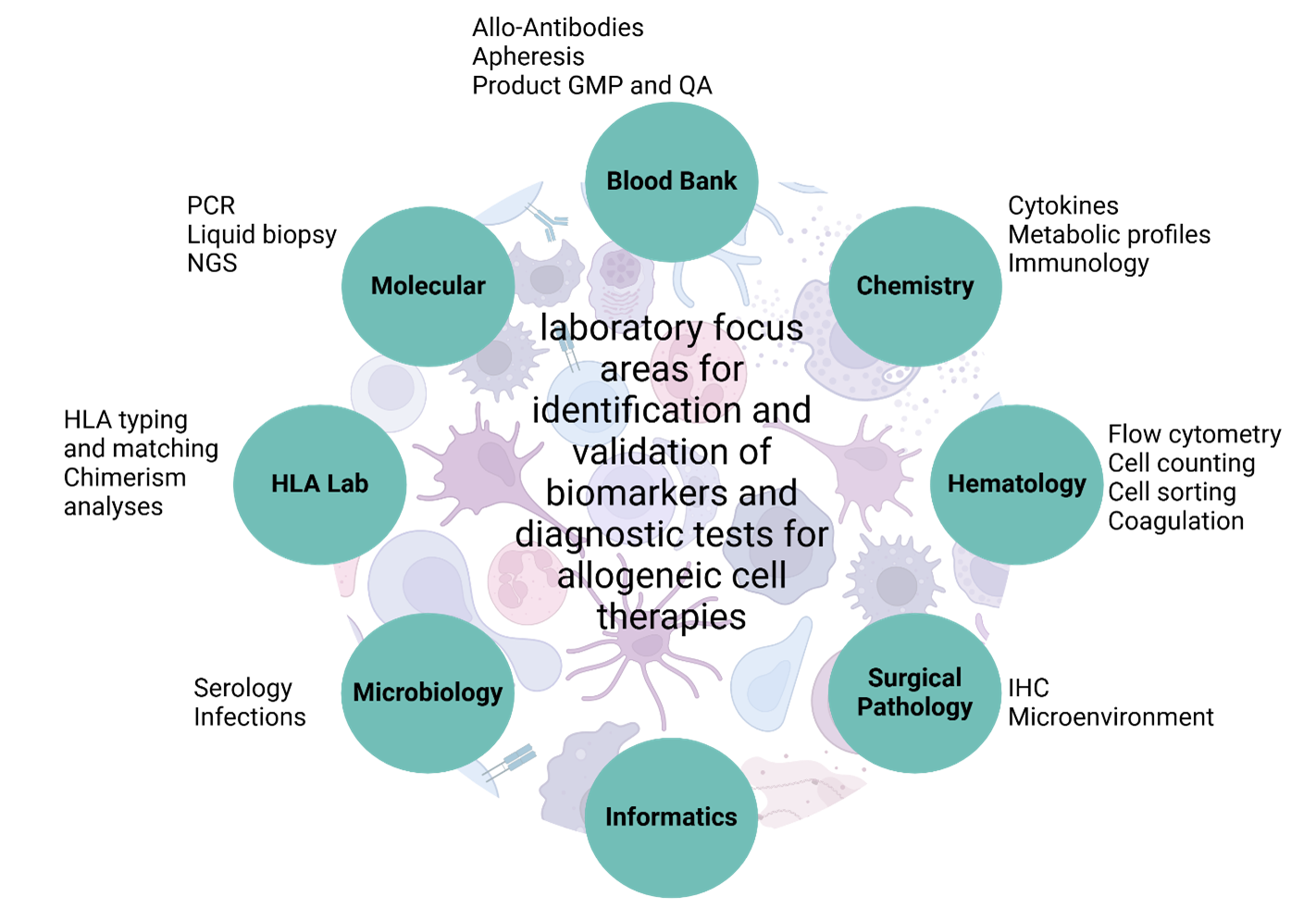
Emerging biomarkers and testing modalities

Figure 1: Potential clinical laboratory focus areas indicated by the currently available literature that may play a role in the development of suitable biomarkers and diagnostic testing.
Considering the rapid innovation and increasing complexity of ACTs, the development of suitable biomarkers and diagnostic testing requires close collaboration between scientists, clinicians, therapeutic developers, and regulatory agencies. Key aspects include the identification of diagnostic, prognostic, and predictive biomarkers to identify complications, provide risk stratification, and assess responsiveness based on a range of information, such as soluble factors, cellular subsets and immunophenotypes, and tumor- and patient-specific genetic signatures. Of note, the exact type and characteristics of specific biomarkers broadly relevant to ACTs are poorly defined. Based on a review of the currently available literature, relevant biomarkers may include integration site and rearrangement analyses, cytokine profiling, proliferation potential, immunogenicity assessment (titers and isotypes of anti-product antibodies), cell enumeration, and phenotypic assessments/immunophenotyping. There will be a need to identify and enumerate cellular products and characterize the surrounding microenvironment in a variety of tissues and fluids, including paucicellular specimens and immune-privileged sites, requiring the development of novel, robust, and clinically feasible and reproducible flow cytometry and IHC panels. Unlike traditional therapies, cell therapies interact dynamically within the patient, necessitating evaluation of the tumor microenvironment and product characteristics in the context of complex and highly specific product kinetics, target cell population levels, and indicators of efficacy and safety. As such, existing diagnostic assays may need to be modified for ACTs. For example, some ACT products may be mixtures of multiple donors, which will require chimerism assays to identify more donors than typically observed in classical HCT. Taken together, it will be necessary for multiple clinical laboratory subspecialties to collaborate and innovate. To identify and validate biomarkers and diagnostic tests for ACTs, there will be a need to integrate cytogenetic and genomic testing; immunophenotyping by flow cytometry and immunohistochemistry for functional and phenotypic characterization; histocompatibility assessment and donor screening based on HLA high-resolution typing and antibody testing; product quality assessment; and emerging technologies and approaches such as omics technologies and machine learning, of which many are not yet available for routine clinical care. Figure 1 summarizes the potential clinical laboratory focus areas that our working group identified that may play a role in the development of suitable biomarkers and diagnostic testing.
Conclusions
While ACTs offer promising off-the-shelf treatment options, addressing challenges in their development and implementation is essential for improving their safety, efficacy, and accessibility to a broader patient population. Although the full range of broadly applicable clinical use cases is still being explored, it is crucial for the pathology and clinical laboratory community to understand the complexity and adaptability of ACT to effectively support ongoing clinical trials and eventual clinical adoption. Identifying key inter-laboratory roles in this evolving field will be a critical first step in developing and advancing diagnostic testing to facilitate the integration of ACT into routine clinical practice.
References
- Challener CA. Moving from Autologous to Allogeneic Cell Therapies: Drivers and Hurdles. January 31, 2023. Accessed December 28, 2024. https://www.pharmasalmanac.com/articles/moving-from-autologous-to-allogeneic-cell-therapies-drivers-and-hurdles
- Mangal A. Scaling Hope: The Growth of Allogeneic Cell Therapy Sector. September 6, 2023. Accessed December 28, 2024. https://www.precisionformedicine.com/blog/article-scaling-hope-the-growth-of-allogeneic-cell-therapy-sector/
- Zhang P, Zhang G, Wan X. Challenges and new technologies in adoptive cell therapy. J Hematol OncolJ Hematol Oncol. 2023;16(1):97.
- Pannu A [@andrewpannu]. Cell therapy landscape. I pulled together 44 companies with a range of approaches & segmented assets by development phase: [Image attached] “[Post].” December 14, 2022. https://x.com/andrewpannu/status/1603940852404871168?mx=2
- Aparicio C, Acebal C, González-Vallinas M. Current approaches to develop “off-the-shelf” chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review. Exp Hematol Oncol. 2023;12(1):73.
- Chen S, van den Brink MRM. Allogeneic “Off-the-Shelf” CAR T cells: Challenges and advances. Best Pract Res Clin Haematol. 2024;37(3):101566.
- Kanate AS, Majhail N, DeFilipp Z, et al. Updated Indications for Immune Effector Cell Therapy: 2023 Guidelines from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2023;29(10):594-597.
- Testa U, Chiusolo P, Pelosi E, Castelli G, Leone G. CAR-T Cell Therapy for T-Cell Malignancies. Mediterr J Hematol Infect Dis. 2024;16(1):e2024031.
- Brudno JN, Maus MV, Hinrichs CS. CAR T Cells and T-Cell Therapies for Cancer: A Translational Science Review. JAMA. 2024;332(22):1924-1935.
- Albelda SM. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol. 2024;21(1):47-66.
- Chung JB, Brudno JN, Borie D, Kochenderfer JN. Chimeric antigen receptor T cell therapy for autoimmune disease. Nat Rev Immunol. 2024;24(11):830-845.
- Ohno R, Nakamura A. Advancing autoimmune Rheumatic disease treatment: CAR-T Cell Therapies - Evidence, Safety, and future directions. Semin Arthritis Rheum. 2024;67:152479.
- Eskandari SK, Daccache A, Azzi JR. Chimeric antigen receptor Treg therapy in transplantation. Trends Immunol. 2024;45(1):48-61.
- Lv Z, Luo F, Chu Y. Strategies for overcoming bottlenecks in allogeneic CAR-T cell therapy. Front Immunol. 2023;14:1199145.
- Andrea AE, Chiron A, Sarrabayrouse G, Bessoles S, Hacein-Bey-Abina S. A structural, genetic and clinical comparison of CAR-T cells and CAR-NK cells: companions or competitors? Front Immunol. 2024;15:1459818.
- Rejeski K, Subklewe M, Aljurf M, et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 2023;142(10):865-877.
- Brudno JN, Kochenderfer JN. Current understanding and management of CAR T cell-associated toxicities. Nat Rev Clin Oncol. 2024;21(7):501-521.
Editorial review and approval of this article was provided by members of the Allogeneic Cell Therapy Project Team (Drs. Matthew Anderson, Gagan Mathur, Jun Zou and Manish Gandhi), a collaboration between the Personalized Healthcare and the Histocompatibility and Identity Testing committees of the College of American Pathologists.

Fabienne Lucas, MD, PhD, FCAP, is an assistant professor in hematopathology at the Department of Laboratory Medicine and Pathology at the University of Washington, Seattle. With a rich background in hemato-oncology, immunology, and flow cytometry, she currently serves the International Clinical Cytometry Society (ICCS) community as DI Committee Chair and Councilor, and the CAP Personalized Health Care Committee (PHC) as a member and co-lead of the allogeneic cell therapy project with Dr. Anderson.

Matthew Anderson, MD, PhD, FCAP
Versiti / Medical Sciences Institute

Manish Gandhi, MD, FCAP
Mayo Clinical Laboratories

Gagan Mathur, MD, MBA, CPE, FCAP
University of California, Irvine

Jun Zou, MD, PhD, FCAP
MD Anderson