- Home
- Advocacy
- Latest News and Practice Data
- June 25, 2024
June 25, 2024
In this Issue:
- How will FDA’s LDT Rule Impact Your Laboratory and Patients? Share Your Story!
- Flawed Special Stains Policy Finally Revised After Years of Persevering Advocacy by the CAP
- FDA Hosts Webinar on IVD Classification July 16
- Register Today! Proposed 2025 Medicare Physician Fee Schedule Webinar
- State Advocacy Win! CAP and DC Pathologists Successfully Advocate to Repeal Laboratory Licensure Law
- Cyberattacks On Health Care Organizations Rise After Change Healthcare Incident
- FDA, International Organizations Issue Artificial Intelligence Guiding Principles
- CAP Urges Pathologists to Respond to AMA-Mathematica Physician Practice Expense Survey ending in June
- Reminder: CAP Encourages Independent Laboratory Practices to Fill Out Survey ending in July
- Take Our News Quiz for June
How will FDA’s LDT Rule Impact Your Laboratory and Patients? Share Your Story!

Do you anticipate the Food and Drug Administration’s (FDA) laboratory-developed test final rule creating unnecessary burdens for pathologists or negatively impacting patients’ lives, innovation, or the economy in your community?
Your story could be used to help legislators understand how the oversight of tests should be based on protecting patients and providing access to safe diagnostic tests with a framework that is the least burdensome for pathologists.
Click here, fill out the form, and tell us more.
Flawed Special Stains Policy Finally Revised After Years of Persevering Advocacy by the CAP
In response to direct advocacy by the CAP, Medicare Administrative Contractors (MACs) Palmetto GBA and Wisconsin Physicians Service updated their Local Coverage Determinations (LCDs) for Special Histochemical Stains and Immunohistochemical Stains (‘special stains’). The policy revisions are the result of extensive advocacy by the CAP and its members over the past ten years, and a direct response to the CAP’s formal reconsideration request initiated in December 2021.
The revised LCDs, which become effective July 14, 2024, expand coverage for Lynch Syndrome tumor screening for microsatellite instability (MSI) / DNA mismatch repair by removing the age limitation, and coverage for IHC for breast pathology to include Ki-67 in a specific population of breast cancer patients. Additional modifications to the LCD coverage criteria allow for greater flexibility for testing in areas like breast, lung, and prostate pathology. Overall, the LCD is less prescriptive and offers clearer actionable guidance for pathologists and other physicians seeking to be compliant in reporting a single service. Further, the updates help to restore physician judgment, as supported by medical evidence.
The special stains LCD covers eight different subspecialties within pathology and is shared by four MACs – Cigna Government Services (CGS), Noridian, Palmetto and Wisconsin Physician Services (WPS). In addition to Palmetto and WPS, the CAP anticipates CGS and Noridian will also adopt the new revisions to their special stains policies.
The revised LCD contains some new documentation requirements for when additional testing is necessary and CAP members should familiarize themselves with the new requirements.
FDA Hosts Webinar on IVD Classification July 16
On July 16 from 1:00 PM – 2:00 PM ET, the Food and Drug Administration (FDA) will host a webinar to discuss how in vitro diagnostic products (IVDs) are classified by the FDA.
IVDs are devices as defined in section 201(h) of the Federal Food, Drug, and Cosmetic Act, and may also be biological products subject to section 351 of the Public Health Service Act. Like other medical devices, IVDs are subject to premarket and post market controls. IVDs are generally also subject to categorization under CLIA.
The FDA classifies medical devices, including IVDs, into Class I, II, or III according to the level of regulatory control that is necessary to reasonably assure safety and effectiveness. The classification of an IVD (or other medical device) determines the appropriate premarket process.
If you have questions that you wish to submit for possible discussion during the webinar, please email CDRHWebinars@fda.hhs.gov.
All questions must be received by June 28, 2024, to be considered for the discussion. Questions will not be taken during the live webinar.
Registration is not necessary. Use this link to join the webinar: https://fda.zoomgov.com/j/1616994355?pwd=cWZhS2RucTU4ZUNLbGF5ZFN5Wlo5dz09
Passcode: %KeTf9
For more information visit: Webinar - In Vitro Diagnostic Product (IVD): Classification
Register Today! Proposed 2025 Medicare Physician Fee Schedule Webinar
The 2025 Medicare Fee Schedule will be released soon! On Tuesday, July 30 at Noon ET/ 11 AM CT, the CAP is offering a complementary live webinar where CAP experts will review proposed 2025 Medicare payment regulations, including the proposed Medicare Physician Fee Schedule and the Quality Payment Program regulations, that will impact Medicare payment for services and pathologists’ participation in the quality initiatives. Webinar presenters will be the Council on Government and Professional Affairs Chair A. Joe Saad, MD, CPE, FCAP; Economic Affairs Committee Chair Ronald McLawhon, MD, PhD, FCAP; and Quality and Clinical Data Registry Affairs Committee Chair Gregary Bocsi, DO, FCAP.
State Advocacy Win! CAP and DC Pathologists Successfully Advocate to Repeal Laboratory Licensure Law
On May 29, District of Columbia Mayor Muriel Bowser enacted a CAP-amended “Health Occupations Revision General Amendment Act of 2023” (B25-0545), successfully repealing DC’s 2015 clinical laboratory licensure law.
The CAP worked closely with the DC Hospital Association (DCHA), the Medical Society of the District of Columbia (MSDC), and numerous DC pathology leaders to strongly urge the repeal of the law. The DC government never operationalized the law following the 2015 enactment. They had several times, most recently this year, attempted implementation.
DC pathologists with CAP support lobbied to repeal the law to address workforce shortages and remove duplicative requirements with CLIA. CAP’s President Donald Karcher, MD, FCAP, previously urged DC City Council to repeal the law during the legislative process. The CAP echoed Dr. Karcher’s comments in a meeting with DC’s Committee on Health.
The act is pending with the US Congress for 30 days ending on July 5, before taking effect as law.
Cyberattacks On Health Care Organizations Rise After Change Healthcare Incident
In April, 44 health care organizations were targeted with ransomware attacks, stolen data, and demand for payment while networks were held hostage. Thirty cases of cyberattacks were reported in March. It is speculated that Change Healthcare’s ransom payment of $22 million may have emboldened cybercriminals to target vulnerable health care organizations, according to research conducted by cybersecurity firm Recorded Future. Cyberattacks against the healthcare sector increased 128% from 2022 to 2023, according to the Office of the Director of National Intelligence.
Other examples of cyberattacks include:
- Earlier in June, pathology firm Synnovis was hit by ransomware, believed to be the work of Russian group Qilin, forcing multiple hospitals in London to delay surgeries and even seek more donations of O-type blood due to the hospitals' inability to match existing blood donations with patients needing transfusions.
- The notorious hacker group LockBit published 61 gigabytes of data stolen from the Simone Veil hospital in Cannes, France, after it refused to pay a ransom.
According to the Department of Health and Human Services (HHS), the health care sector is particularly vulnerable to cybersecurity risks. Health care facilities are attractive targets for cyber criminals in light of their size, technological dependence, sensitive data, and unique vulnerability to disruptions.
- Review the latest cybersecurity federal alerts and advisories.
- Access educational materials designed by HHS to give HIPAA covered entities and businesses insight into how to respond to a cyber-related security incident.
- Access American Medical Association (AMA) resources for physicians and health care staff to protect patient health records and other data from cyberattacks.
Make sure to check out our new resource: Cyberattacks and Cybersecurity in Health Care and check back for the latest information and updates.
FDA, International Organizations Issue Artificial Intelligence Guiding Principles
On June 13, the FDA, Health Canada, and the United Kingdom’s Medicines and Healthcare products Regulatory Agency jointly issued the Transparency for Machine Learning-Enabled Medical Devices: Guiding Principles. In 2021, the groups jointly identified ten guiding principles for good machine learning practice. The guiding principles released June 13 build upon the 2021 principles. These principles support the development of safe, effective, and high-quality artificial intelligence/machine learning technologies that can be learned from real-world use and, in some cases, improve device performance.
They further identified guiding principles for transparency for machine learning-enabled medical devices which build upon the 2021 principles. Effective transparency is as follows:
- ensures that information that could impact risks and patient outcomes is communicated.
- considers the information that the intended user or audience needs and the context in which it's used.
- uses the best media, timing, and strategies for successful communication,
- relies on a holistic understanding of users, environments, and workflows.
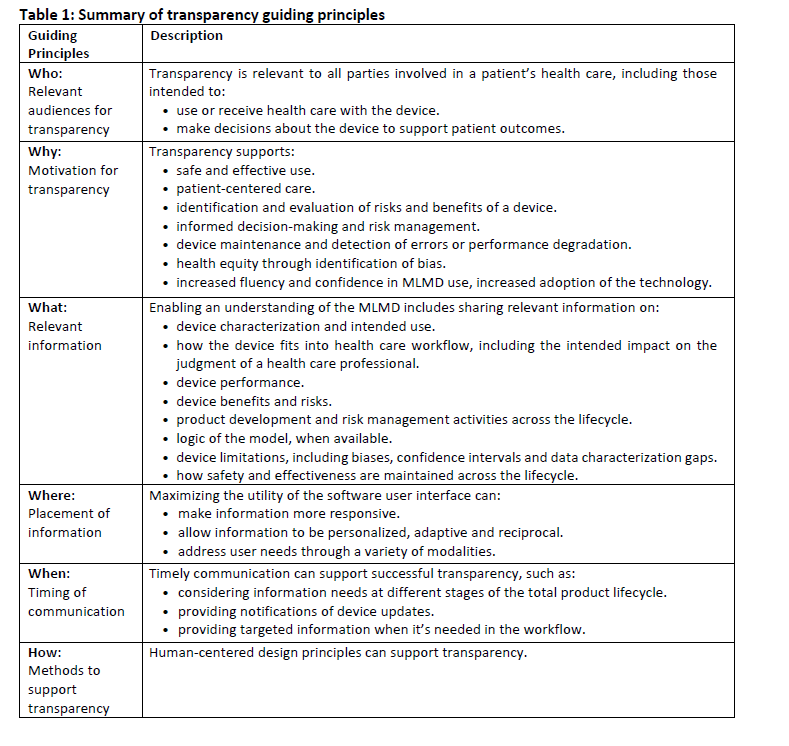
These guiding principles are intended as considerations when adopting and advancing good transparency practices.
In prior advocacy to the FDA and the ONC, the CAP has supported transparency in AI/ML devices, noting that transparency must be required in any regulatory framework, which should mandate that developers implement an open system that describes updates and modifications as they occur to patients and clinicians.
CAP Urges Pathologists to Respond to AMA-Mathematica Physician Practice Expense Survey ending in June
The CAP is one of more than 170 health care organizations supporting a national study by the AMA and Mathematica that will collect representative data on physician practice expenses. The aim of the AMA Physician Practice Information Survey is to better understand the costs faced by today’s physician practices to support physician payment advocacy.
Pathologists and their practices must watch for invitations to complete the survey. Invitations and reminders about the costs survey will come from PPISurvey@mathematica-mpr.com. Invitations and reminders about physician hours worked will come from
PhysicianHoursSurvey@mathematica-mpr.com with the subject line: “Please help to update accurate physician payments.” Your input will ensure future pathology payment rates are accurate.
The study will serve as an opportunity to communicate accurate financial information to policymakers, including members of Congress and the Centers for Medicare & Medicaid Services (CMS). The survey has been extended through at least the end of June.
Physicians will be randomly selected to participate. If contacted, you will receive a $100 stipend for participating in the survey and your individual practice data will be kept private. Participation is voluntary but critical to the success of efforts to support accurate resource-based physician payment.
Again, the CAP strongly urges all physicians who are selected for the surveys to respond as soon as possible. For more information read the Physician Practice Information Survey Methodology Report.
Reminder: CAP Encourages Independent Laboratory Practices to Fill Out Survey ending in July
The data firm Mathematica has sent out a national survey of independent laboratory practices to collect updated and accurate data on practice and laboratory costs which are a key element of physician fee schedule payment for pathology services. The survey results will be used by the Centers for Medicare and Medicaid Services for its physician fee schedule payment methodology. These data have not been updated since last collected over 15 years ago and it is critically important to update these data to ensure accurate payment.
This survey, endorsed by over 170 other medical societies and associations, will serve as a supplement to the American Medical Association led Physician Practice Information Survey.
The CAP contracted with Mathematica, an independent research company with extensive experience in survey methods and health care delivery and practice costs, to conduct this survey.
Independent laboratory practices will be randomly selected to participate. The survey is focused on collecting financial information and should be completed by the person(s) at the clinician practice who can best answer questions about finances and expenses. The time it takes to complete this survey will vary depending on the size and complexity of the practice. It is critical that this survey be completed to ensure that the needed updates are made to practice expenses and costs used to ensure accurate Medicare payment.
To thank practices for their participation, Mathematica will send a summary report that compares their data with national averages. The information shared will be kept private, reported in aggregate, and used only to inform the national estimates of practice expense per patient care hour. If you have any questions, please contact Mathematica at CPISurvey@mathematica-mpr.com, or by phone at 1-833-714-0022.
Take Our News Quiz for June
Are you up to speed on CAP advocacy news? Take our new monthly news quiz and see how many you can get right and share your results on social media.